 |
Anti-VEGF therapy has been very effective in disrupting the progression of neovascular AMD (nAMD) and even reversing vision loss in many cases. However, non-nAMD, characterized by regions of irreversible RPE cell loss, or geographic atrophy (GA), slowly results in severe and irreversible vision loss. The Age-Related Eye Disease Study (AREDS) showed progression from intermediate to non-nAMD takes about 2.5 to five years.1 To date, no effective treatment exists for non-nAMD.
The exact etiology of AMD is unknown, but a combination of genetic and environmental factors have been implicated.2,3 While the primary cause of acute vision loss in nAMD is choroidal neovascularization (CNV), RPE loss and geographic atrophy are final common endpoints for both nAMD and non-nAMD.
Questions Stem Cell-based Therapy Must Answer
The RPE monolayer is essential both for the survival of photoreceptors and the underlying choriocapillaris. Among other functions, the RPE secretes pigment epithelial-derived factor (PEDF), vascular endothelial growth factor (VEGF) as well as the extracellular matrix which may have an antiangiogenic function.
For this reason, any stem cell-based therapy must answer several questions, among them:
-Will the donor RPE survive long enough to justify surgical risks?
-Will the donor RPE maintain its polarity and function; that is, will donor RPE form functional synaptic connections with host photoreceptor neurons and facilitate the visual cycle?
-Can the donor RPE reverse or prevent further degeneration?
-Are sources of donor RPE ethically available?
-What is the best surgical technique?
While RPE
| Focus for the Future Of Stem Cell Research • Development of novel, noninvasive diagnostic tests to assay retinal pigment epithelium and retinal function at the molecular and cellular level. • Development of novel surgical tools and surgical methods for optimal delivery of RPE. • Advancement of stem-cell science for the purpose of understanding host retinal immune response. • Development of clinical-grade methods to genetically modify stem cell-derived RPE. • Development of rehabilitation strategies for training patients to use optimum fixation viewing techniques with transplanted stem cells for visually guided tasks to enhance function. |
Investigators demonstrated proof-of-principle decades ago that RPE transplantation could work in human subjects and animal models.4–6 These studies were complemented by macular surgeries that demonstrated that translocating the fovea over an apparently normal region of RPE allowed short-term visual gains.7 Unfortunately, long-term follow-up showed high GA recurrence rates in the new subfoveal RPE region.8,9
Other sources of cells have been used as donor RPE including homologous, heterologous or autologous adult RPE transplantation as well as fetal RPE transplantation.8,10-14
Attempts at human RPE transplantation in GA using autologous and allogeneic RPE have had limited success but strongly suggest that RPE replacement strategies can work if the limitations associated with the cell sources and surgical methods could be overcome.15-20
Sources for Stem Cells
Investigators have reported several hundred RPE or stem cell-based grafts since the first human homologous and autologous RPE transplantation in 1991, and this number is growing.16,21,22 Some of these studies use non-RPE stem-cell populations, such as bone marrow-derived stem cells, delivered with either intravitreal or intravenous adminstration.23 The predominant mechanism of action of these studies is through nonspecific trophic effects of stem cells to support retinal and RPE function. Interestingly, evidence has shown RPE repopulation by systemic administration of some bone marrow-derived cell lines in animal models,24,25 but the safety and efficacy of systemic administration seems more problematic than with local delivery methods, which are also viable and very well developed.
Among the numerous sources of donor RPE, induced pluripotent stem cells (iPSC) and human embryonic stem cells (hESC) are the most feasible. Both stem cell forms are a source of potentially endless RPE donor cells that can be fully differentiated into RPE either as cell suspension or monolayers.
| Take-home Point The quest to restore sight from several blinding retinal diseases has shown promise through groundbreaking research emerging from stem cell-based therapies. Subretinal transplantation of autologous or allogeneic retinal pigment epithelium (RPE) over the past several decades has shown that stem cell-derived RPE can rescue photoreceptor function and improve some aspects of visual function in animal models and in human subjects with neovascular age-related macular degeneration and non-neovascular AMD. Thanks to advances in stem-cell biology and regenerative medicine, new methods of differentiating RPE from human embryonic stem cells (hESC) and induced pluripotent stem cells (iPSC) can provide a potentially unlimited supply of RPE cells. Parallel advances in noninvasive retinal imaging and vitreoretinal surgery now provide the tools to effectively identify relevant anatomic changes with functional correlations in vivo and deliver stem cell-based therapies. This review focuses on the potential and challenges of stem cell-based treatments for AMD. |
• iPSC. Induced pluripotent stem cells are derived from fully differentiated adult somatic cells that are reprogrammed in vitro to differentiate into RPE.25 They perform phagocytic functions, demonstrate RPE-like gene expression profiles and promote photoreceptor survival.26–28 A Japanese study that used autologous iPSC RPE cells for AMD therapy was recently stopped due to unexpected mutations (three aberrations in DNA copy number),27,29 and was suspended because of concerns about the possible effects the deletions could have.30.31
• hESC. In contrast, human embryonic stem cells are derived from the inner cell mass of blastocysts and can also be programmed to differentiate into RPE.31 Manufacture of these cells can undergo strict
quality-control testing and avoid the complicated and high-risk process of surgically harvesting autologous or allogeneic grafts.
Lastly, both
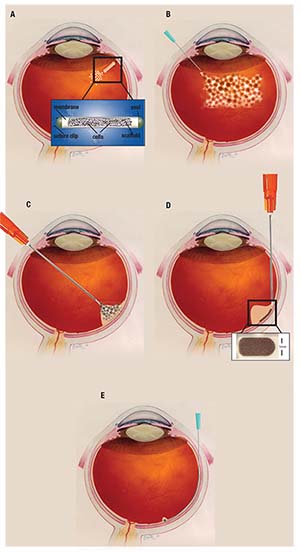 |
| Figure. Methods for delivering stem cells include: A) intraocular encapsulated cell technology via insert; B) intravitreal injection; C) pars plana vitrectomy with subretinal cell suspension injection via cannula; D) pars plana vitrectomy with subretinal graft placement via cannula; and E) subretinal delivery of stem-cell suspension. |
Regardless of the stem cell source, transplanted RPE cells will likely require some form of mechanical or physical substrate because RPE survival depends highly on extracellular substrates.32,33 For example, a healthy Bruch’s membrane has been shown to improve the survival, repopulation and confluence of RPE cells.34
Such a substrate must not only support RPE attachment and differentiation; it must also be amenable to surgical manipulation and be immunologically silent. While donor RPE in suspension may attach to exogenous Bruch’s membrane, they more commonly aggregate in multiple layers and assume an abnormal phenotype.35
In addition, dissociated hESC-derived RPE can dedifferentiate and may not redifferentiate appropriately. Groups have developed various scaffolds to support RPE survival and implantation, including biodegradable scaffolds and biologically inert but nondegradable scaffolds such as polyester membranes, plasma polymers, polyimide and parylene.20,36–39 Researchers have shown that subretinal implantation of human embryonic stem cell-derived RPE monolayers on a parylene substrate have improved survival compared to cell suspensions.40,41
Evaluating the Host Tissue
Detailed assessment coupling macular anatomical and functional correlations are critical for assessing the viability of a subject’s retina for stem cell-based rehabilitation and for postoperative assessment of
success.
Spectral-domain
 |
Subjects with such severe and chronic anatomical changes may not show significant improvement in visual function under any circumstances. It is possible that RPE transplantation in this population may preserve the remaining RPE and retina at the borders of geographic atrophy lesions or slow the progression of disease.
It is exciting to speculate about stem-cell therapy as a replacement for neurosensory retina, specifically photoreceptor cells, either alone or in addition to RPE transplantation, although this is not currently in any clinical trial. Since most cases of severe non-nAMD ultimately involve loss of photoreceptors, it is likely that such a treatment will be necessary for severe disease.
Interestingly, transplantation of human fetal retina into nude adult rat retina has resulted in histologically detectable synaptic connections.44 Adult
 |
Stem-Cell Delivery Techniques
Regardless of the source of RPE, the delivery techniques include intravitreal injection, subretinal injection of cell suspension of RPE or subretinal placement of a sheet of tissue containing RPE (Figure D).49
Injection of cell suspensions into the subretinal space is advantageous because it does not require a large retinotomy and is relatively fast and simple.50 Major limitations of this method include the reflux of RPE cells into the vitreous, relatively poor adherence to Bruch’s membrane and failure to form an effective monolayer.36,51
Alternatively, the delivery of subretinal sheets containing RPE has also been demonstrated but requires larger retinotomies, takes longer and is prone to incorrect implant orientation and more postoperative complications.36,52
The main advantage of implanting RPE sheets with a scaffold is that the orientation, polarization and function of the RPE is more likely to consistently replicate that of the native RPE.
Despite the limitations of both methods, current clinical trials hold some promise.22,44,52,53 In some cases cells can be implanted in a self-contained and nonimmunogenic manner that allows the secretion of trophic factors but isolates the cells from the host environment. RS
The authors acknowledge the California Institute for Regenerative Medicine (CIRM) and Research to Prevent Blindness (RPB) for grant support.
REFERENCES
1. Rudnicka AR, Kapetanakis VV, Jarrar Z, et al. Incidence of late-stage age-related macular degeneration in American whites: systematic review and meta-analysis. Am J Ophthalmol. 2015;160:85-93 e83.
2. Ferris FL, 3rd, Wilkinson CP, Bird A, et al. Clinical classification of age-related macular degeneration. Ophthalmology. 2013;120:844-851.
3. Klein R, Meuer SM, Knudtson MD, Klein BE. The epidemiology of progression of pure geographic atrophy: the Beaver Dam Eye Study. Am J Ophthalmol. 2008;146:692-699.
4. Miller JW. Age-related macular degeneration revisited--piecing the puzzle: the LXIX Edward Jackson memorial lecture. Am J Ophthalmol. 2013;155:1-35 e13.
5. Gouras P, Flood MT, Kjedbye H, Bilek MK, Eggers H. Transplantation of cultured human retinal epithelium to Bruch’s membrane of owl monkey’s eye. Curr Eye Res. 1985;4:253-265.
6. Gouras P, Lopez R, Kjeldbye H, Sullivan B, Brittis M. Transplantation of retinal epithelium prevents photoreceptor degeneration in the RCS rat. Prog Clin Biol Res. 1989;314:659-671.
7. Li LX, Turner JE. Inherited retinal dystrophy in the RCS rat: prevention of photoreceptor degeneration by pigment epithelial cell transplantation. Exp Eye Res. 1988;47:911-917.
8. Cahill MT, Freedman SF, Toth CA. Macular translocation with 360 degrees peripheral retinectomy for geographic atrophy. Arch Ophthalmol. 2003;121:132-133.
9. Benner JD, Sunness JS, Ziegler MD, Soltanian J. Limited macular translocation for atrophic maculopathy. Arch Ophthalmol. 2002;120:586-591.
10. Cahill MT, Mruthyunjaya P, Bowes Rickman C, Toth CA. Recurrence of retinal pigment epithelial changes after macular translocation with 360 degrees peripheral retinectomy for geographic atrophy. Arch Ophthalmol. 2005;123:935-938.
11. Peyman GA, Blinder KJ, Paris CL, Alturki W, Nelson NC, Jr., Desai U. A technique for retinal pigment epithelium transplantation for age-related macular degeneration secondary to extensive subfoveal scarring. Ophthalmic Surg. 1991;22:102-108.
12. Stanga PE, Kychenthal A, Fitzke FW, et al. Retinal pigment epithelium translocation after choroidal neovascular membrane removal in age-related macular degeneration. Ophthalmology. 2002;109:1492-1498.
13. Binder S, Krebs I, Hilgers RD, et al. Outcome of transplantation of autologous retinal pigment epithelium in age-related macular degeneration: a prospective trial. Invest Ophthalmol Vis Sci. 2004;45:4151-4160.
14. van Meurs JC, Van Den Biesen PR. Autologous retinal pigment epithelium and choroid translocation in patients with exudative age-related macular degeneration: short-term follow-up. Am J Ophthalmol. 2003;136:688-695.
15. Durlu YK, Tamai M. Transplantation of retinal pigment epithelium using viable cryopreserved cells. Cell Transplant. 1997;6:149-162.
16. Algvere PV, Gouras P, Dafgard Kopp E. Long-term outcome of RPE allografts in non-immunosuppressed patients with AMD. Eur J Ophthalmol. 1999;9:217-230.
17. Bhatt NS, Newsome DA, Fenech T, et al. Experimental transplantation of human retinal pigment epithelial cells on collagen substrates. Am J Ophthalmol. 1994;117:214-221.
18. Sheng Y, Gouras P, Cao H, et al. Patch transplants of human fetal retinal pigment epithelium in rabbit and monkey retina. Invest Ophthalmol Vis Sci. 1995;36:381-390.
19. Joussen AM, Joeres S, Fawzy N, et al. Autologous translocation of the choroid and retinal pigment epithelium in patients with geographic atrophy. Ophthalmology. 2007;114:551-560.
20. Caramoy A, Liakopoulos S, Menrath E, Kirchhof B. Autologous translocation of choroid and retinal pigment epithelium in geographic atrophy: long-term functional and anatomical outcome. Br J Ophthalmol. 2010;94:1040-1044.
21. Algvere PV, Berglin L, Gouras P, Sheng Y, Kopp ED. Transplantation of RPE in age-related macular degeneration: observations in disciform lesions and dry RPE atrophy. Graefes Arch Clin Exp Ophthalmol. 1997;235:149-158.
22. Weisz JM, Humayun MS, De Juan E, Jr., et al. Allogenic fetal retinal pigment epithelial cell transplant in a patient with geographic atrophy. Retina. 1999;19:540-545.
23. da Cruz L, Chen FK, Ahmado A, Greenwood J, Coffey P. RPE transplantation and its role in retinal disease. Prog Retin Eye Res. 2007;26:598-635.
24. Park SS, Bauer G, Abedi M, et al. Intravitreal autologous bone marrow CD34+ cell therapy for ischemic and degenerative retinal disorders: preliminary phase 1 clinical trial findings. Invest Ophthalmol Vis Sci. 2015;56:81-89.
25. Harris JR, Brown GA, Jorgensen M, et al. Bone marrow-derived cells home to and regenerate retinal pigment epithelium after injury. Invest Ophthalmol Vis Sci. 2006;47:2108-2113.
26. Buchholz DE, Hikita ST, Rowland TJ, et al. Derivation of functional retinal pigmented epithelium from induced pluripotent stem cells. Stem Cells. 2009;27:2427-2434.
27. Carr AJ, Vugler AA, Hikita ST, et al. Protective effects of human iPS-derived retinal pigment epithelium cell transplantation in the retinal dystrophic rat. PLoS One. 2009;4:e8152.
28. Kamao H, Mandai M, Wakamiya S, et al. Objective evaluation of the degree of pigmentation in human induced pluripotent stem cell-derived RPE. Invest Ophthalmol Vis Sci. 2014;55:8309-8318.
29. Kamao H, Mandai M, Okamoto S, et al. Characterization of human induced pluripotent stem cell-derived retinal pigment epithelium cell sheets aiming for clinical application. Stem Cell Reports. 2014;2:205-218.
30. Ilic D. iPSC in the past decade: the Japanese dominance. Regen Med. 2016;11:747-749.
31. Mandai M, Watanabe A, Kurimoto Y, et al. Autologous induced stem-cell-derived retinal cells for macular degeneration. N Engl J Med. 2017;376:1038-1046.
32. Rowland TJ, Blaschke AJ, Buchholz DE, Hikita ST, Johnson LV, Clegg DO. Differentiation of human pluripotent stem cells to retinal pigmented epithelium in defined conditions using purified extracellular matrix proteins. J Tissue Eng Regen Medicine. 2013;7:642-653.
33. Rowland TJ, Buchholz DE, Clegg DO. Pluripotent human stem cells for the treatment of retinal disease. J Cell Physiol. 2012;227:457-466.
34. Tezel TH, Del Priore LV. Reattachment to a substrate prevents apoptosis of human retinal pigment epithelium. Graefes Arch Clin Exp Ophthalmol. 1997;235:41-47.
35. Tezel TH, Kaplan HJ, Del Priore LV. Fate of human retinal pigment epithelial cells seeded onto layers of human Bruch’s membrane. Invest Ophthalmol Vis Sci. 1999;40:467-476.
36. Schwartz SD, Regillo CD, Lam BL, et al. Human embryonic stem cell-derived retinal pigment epithelium in patients with age-related macular degeneration and Stargardt’s macular dystrophy: follow-up of two open-label phase 1/2 studies. Lancet. 2015;385:509-516.
37. Stanzel BV, Liu Z, Brinken R, Braun N, Holz FG, Eter N. Subretinal delivery of ultrathin rigid-elastic cell carriers using a metallic shooter instrument and biodegradable hydrogel encapsulation. Invest Ophthalmol Vis Sci. 2012;53:490-500.
38. Zuber AA, Robinson DE, Short RD, Steele DA, Whittle JD. Development of a surface to increase retinal pigment epithelial cell (ARPE-19) proliferation under reduced serum conditions. J Mater Sci Mater Med. 2014;25:1367-1373.
39. Subrizi A, Hiidenmaa H, Ilmarinen T, et al. Generation of hESC-derived retinal pigment epithelium on biopolymer coated polyimide membranes. Biomaterials. 2012;33:8047-8054.
40. Diniz B, Thomas P, Thomas B, et al. Subretinal implantation of retinal pigment epithelial cells derived from human embryonic stem cells: improved survival when implanted as a monolayer. Invest Ophthalmol Vis Sci. 2013;54:5087-5096.
41. Lu B, Zhu D, Hinton D, Humayun MS, Tai YC. Mesh-supported submicron parylene-C membranes for culturing retinal pigment epithelial cells. Biomed Microdevices. 2012;14:659-667.
42. Hu Y, Liu L, Lu B, et al. A novel approach for subretinal implantation of ultrathin substrates containing stem cell-derived retinal pigment epithelium monolayer. Ophthalmic Res. 2012;48:186-191.
43. Sayegh RG, Kiss CG, Simader C, et al. A systematic correlation of morphology and function using spectral domain optical coherence tomography and microperimetry in patients with geographic atrophy. Br J Ophthalmol. 2014;98:1050-1055.
44. Joussen AM, Heussen FM, Joeres S, et al. Autologous translocation of the choroid and retinal pigment epithelium in age-related macular degeneration. Am J Ophthalmol. 2006;142:17-30.
45. Aramant RB, Seiler MJ. Fiber and synaptic connections between embryonic retinal transplants and host retina. Exp Neurol. 1995;133:244-255.
46. Kaplan HJ, Tezel TH, Berger AS, Wolf ML, Del Priore LV. Human photoreceptor transplantation in retinitis pigmentosa. A safety study. Arch Ophthalmol. 1997;115:1168-1172.
47. Humayun MS, de Juan E, Jr., del Cerro M, et al. Human neural retinal transplantation. Invest Ophthalmol Vis Sci. 2000;41:3100-3106.
48. Das T, del Cerro M, Jalali S, et al. The transplantation of human fetal neuroretinal cells in advanced retinitis pigmentosa patients: results of a long-term safety study. Exp Neurol. 1999;157:58-68.
49. Radtke ND, Aramant RB, Seiler M, Petry HM. Preliminary report: indications of improved visual function after retinal sheet transplantation in retinitis pigmentosa patients. Am J Ophthalmol. 1999;128:384-387.
50. Yu DY, Cringle SJ, Alder VA, Su EN. Intraretinal oxygen distribution in rats as a function of systemic blood pressure. Am J Physiol. 1994;267:H2498-2507.
51. Binder S, Stolba U, Krebs I, et al. Transplantation of autologous retinal pigment epithelium in eyes with foveal neovascularization resulting from age-related macular degeneration: A pilot study. Am J Ophthalmol. 2002;133:215-225.
52. Gouras P, Algvere P. Retinal cell transplantation in the macula: new techniques. Vision Res. 1996;36:4121-4125.
53. Heussen FM, Fawzy NF, Joeres S, et al. Autologous translocation of the choroid and RPE in age-related macular degeneration: 1-year follow-up in 30 patients and recommendations for patient selection. Eye (Lond). 2008;22:799-807.
54. Schwartz SD, Tan G, Hosseini H, Nagiel A. Subretinal transplantation of embryonic stem cell-derived retinal pigment epithelium for the treatment of macular degeneration: An assessment at 4 years. Invest Ophthalmol Vis Sci. 2016;57:ORSFc1-9.
55. Ho AC, Chang TS, Samuel M, Williamson P, Willenbucher RF, Malone T. Experience with a subretinal cell-based therapy in patients with geographic atrophy secondary to age-related macular degeneration. Am J Ophthalmol. 2017;179:67-80.



