| ABOUT THE AUTHORS |
 Dr. Gupta is an assistant professor of ophthalmology at Weill Cornell Medical College/New York–Presbyterian Hospital. Dr. Gupta is an assistant professor of ophthalmology at Weill Cornell Medical College/New York–Presbyterian Hospital. |
 Dr. Chan is a professor of ophthalmology and visual sciences at Illinois Eye and Ear Infirmary, University of Illinois at Chicago. Dr. Chan is a professor of ophthalmology and visual sciences at Illinois Eye and Ear Infirmary, University of Illinois at Chicago. |
| DISCLOSURES: Dr. Chan is a consultant for Alcon and Allergan. |
Advances in Retinal Imaging
The traditional approach to documenting retinal pathology in children involved clinical examination, sometimes under anesthesia, and retinal drawings. Advances in imaging have dramatically improved our ability to capture images of the pediatric retina either at bedside or in the office. In contrast to the often brief view indirect ophthalmoscopy provides, retinal photography allows us to examine retinal features in greater detail and to assess subtle changes between visits better than fundus drawings could.
Widefield fundus cameras such as the RetCam (Clarity Medical Systems) enable handheld imaging and fluorescein angiography of infants in the clinic and at bedside. RetCam achieves 130° of mydriatic retinal imaging using a handheld contact camera system with a fiberoptic cable. While well-suited for retinopathy of prematurity, use of the camera in older infants and children typically requires anesthesia. Additionally, imaging of the far periphery requires peripheral sweeps or montaging.
The Optomap (Optos) ultra-widefield imaging is a non-contact, nonmydriatic system that allows rapid, office-based photography and FA of a wider retinal arzttea (200°). Optomap imaging has been reported in patients as young as 5 years with a number of pediatric retinal conditions,1,2 and we have used it on some children as young as 3 years (Figure 1, page 16). Additionally, Optomap ultra-widefield FA-guided panretinal photocoagulation has been reported in patients with conditions such as Coats’ disease and familial exudative vitreoretinopathy.1 Recent studies describe a “flying baby” technique of holding premature infants upright onto the Optomap interface for ROP imaging,3 but the technique is technically challenging and typically does not work in babies older than 6 months.4
The Heidelberg Spectralis ultra-widefield imaging module (Heidelberg Engineering) uses a camera attachment to capture non-contact-based, high-contrast images of up to 102° of the retina. This system has been used for imaging of babies under anesthesia at 33 weeks gestational age through 12 months. The lens can be rotated 90° for imaging in supine patients.4 While less portable than the RetCam and with a smaller widefield than Optomap, Spectralis offers the ability to use additional modalities such as optical coherence tomography and indocyanine green angiography.
In the current pediatric retinal imaging paradigm, very young infants such as neonates undergoing ROP screenings can be imaged using the RetCam system, while children 3-5 years of age and older can often cooperate with office-based Optomap imaging. Imaging of older infants and children younger than 3-5 years generally requires examination under anesthesia, usually with the RetCam.
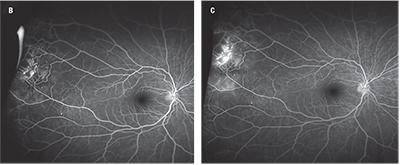
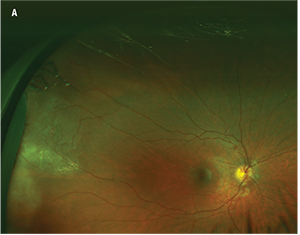 Figure 1. Fundus photography of an 11-year-old patient with Coats’ disease reveals temporal exudation and telangiectasias (A). Fluorescein angiography reveals light-bulb telangiectasias (B at 1 minute, 23 seconds; C at 5 minutes, 18 seconds). |
 |
The PanoCam LT (Visunex Medical Systems) widefield handheld system is now approved in the United States and offers a more compact, wireless, contact-based system for newborn widefield imaging (130°).
The 3nethra Classic (Forus Health) is a compact, table-top, non-mydriatic digital imaging system offering a 40-45° field of view. The 3nethra neo is a lightweight, non-wireless but compact, contact-based system for high-definition imaging with a 120° field of view. This system is well-suited for ROP imaging and offers montaging capabilities. Both Forus systems are affordably priced, compact and enable secure transmission of images to a cloud-based system, making them particularly well-suited for telemedicine.
Impact of Widefield Imaging
The recent widespread availability of widefield imaging systems with FA capabilities such as the RetCam has brought to light the utility of FA for ROP diagnosis. Recent studies have suggested that compared to color fundus photographs alone, FA resulted in increased sensitivity for diagnosis of stage 2 or worse ROP, stage 3 or worse ROP, pre-plus or worse disease and type 2 or worse ROP,5 but did not improve diagnosis of the macular center and only marginally improved sensitivity for zone diagnosis.6 These findings have important implications for the use of FA in ROP surveillance as well as in remote, imaging-based, telemedicine screening.
Based largely on FA features and clinical course, Audina Berrocal, MD, and colleagues have described ROPER (ROP vs. FEVR), in premature infants who exhibit retinal findings more characteristic of FEVR than ROP. The distinguishing FA features in ROPER include irregular sprouts of vascularization at the vascular/avascular junction (vs. the more uniform advancing front of ROP), distinct pruning of vessels, pinpoint areas of hyperfluorescence and segmental areas of vascular leakage.7 The diagnosis of ROPER may have implications for management because, like FEVR, these patients may exhibit a more unpredictable and long-term course than those with ROP.
| Take-home Point Insights gleaned from advances in pediatric retinal imaging have impacted our understanding and diagnosis of pediatric retinal disease. These advances, coupled with tools for computer-based image analysis, also have implications for telemedicine. These imaging advances, along with the potential of pharmacologic vitreolysis and the growing role of anti-VEGF agents are moving forward our ability to treat pediatric retinal disease. |
They reported that these findings have implications for the classification and prognosis of FEVR. For example, widefield imaging revealed a subset of stage 2 FEVR patients who exhibited fluorescein leakage without clinical exudation (stage 2a in the original scheme), and this population seemed to be at high risk of progression to retinal detachment (stage 3).8 The proposed revised staging system includes angiographic leakage, even in the absence of clinical exudate, as a criterion for stage 2b FEVR. Similarly, widefield angiography revealed that some patients with early FEVR exhibit anomalous intraretinal vascular patterns rather than an avascular retinal periphery, so these features would be included in the revised stage 1. A subset of the stage 1 patients also exhibited angiographic leakage, which can result in exudation limiting the ability to treat. The revised stage 1 is now sub-categorized as stage 1a and 1b, reflecting those without or with clinical exudate or fluorescein leakage. This proposed re-classification has therapeutic implications, as the authors recommend treatment for the now-broadened stage 2b and the newly conceived stage 1b FEVR.8
In a separate study, Dr. Trese and colleagues found that up to 58 percent of asymptomatic relatives of individuals who have FEVR exhibited subtle stage 1 and 2 FEVR features on widefield FA, and 35 percent exhibited stage 2 FEVR. This led them to recommended clinical and widefield imaging screening of family members of patients with FEVR.9
Potential for Telemedicine
Advances in retinal imaging and image analysis tools have complemented the development of telemedicine in retina, particularly for ROP. In the United States, only 29 percent of neonatal intensive care units provide both ROP diagnosis and treatment capabilities,10 and the need is even more striking worldwide.
| Ocular Manifestations of the Zika Virus Recent months have seen a rapid spread of the Zika virus (ZIKV) through Brazil and elsewhere in the Americas, and ZIKV infection in pregnant women is thought to potentially be associated with microcephaly in the infants born to these women. A recent study on 29 infants with microcephaly and presumed congenital ZIKV infection revealed a high prevalence of ocular abnormalities (17 eyes [29.3 percent] of 10 children [34.5 percent]).32 These abnormalities included retinal and chorioretinal atrophy in 64.7 percent of eyes, optic nerve abnormalities (47.1 percent), bilateral iris coloboma (11.8 percent) and lens subluxation (5.9 percent). This high prevalence of severe ocular abnormalities in infants with presumed congenital ZIKV infection not only suggests a need for routine ophthalmologic evaluation in all infants with potential congenital ZIKV infection, but also poses a significant public-health concern. |
To facilitate telemedicine-based ROP surveillance, the Imaging and Informatics in Retinopathy of Prematurity (i-ROP) consortium has created a database of all ROP infants screened at 12 major academic institutions worldwide.15 The large repository of images in the database, in combination with their “reference standard diagnosis,”15 has become a major research tool in ROP.16 Approximately 2,500 image sets and “reference standard diagnoses” from this database have been used in a collaboration of the i-ROP consortium and the Global Education Network for Retinopathy of Prematurity (GEN-ROP) to create a web-based ROP tele-educational system that has been validated in the ophthalmology resident population to improve accuracy of ROP diagnosis.17 This validation tool may have utility in certification of telemedicine experts and in training ophthalmologists.
A major challenge in ROP diagnosis is lack of agreement amongst experts on diagnosis of plus disease.18 The ROPTool is a computer program that traces retinal vessels to quantify vascular dilation and tortuosity. Studies have suggested that the ROPTool and other available image analysis tools can aid in the diagnosis of plus disease.19
| Newer Instrumentation and Pediatric Vitreoretinal Surgery The pediatric eye differs significantly from the adult eye because it is not only smaller, but the lens is relatively large and intraocular structures relatively closer in proximity. Moreover, the sclera is thinner and potentially more prone to sclerotomy leaks, the non-syneretic vitreous is thicker, and posterior vitreous detachment induction can be challenging. The Alcon 25+ gauge short instruments are shorter and stiffer than standard instruments. These short instruments may be better-suited for the smaller space of the pediatric eye and confer theoretically less risk of lens trauma. The stiffness of the instruments can enable surgical maneuvers through thin sclera and thick vitreous with potentially less risk of instrument bending. The 25+ gauge short packs come with a sutured-in infusion. The utility of 27-gauge instrumentation with faster cut rates (7,500) for pediatric retinal surgery remains to be determined. The smaller instrumentation may be well-suited for smaller eyes and have less risk of causing postoperative sclerotomy leaks, but the greater flexibility of the 27-gauge system may pose some challenges. It is also unclear whether the higher cut rates will efficiently cut the pediatric vitreous. Further studies of the 27-gauge systems in pediatric vitreoretinal surgery are needed. |
Beyond ROP, interest in universal telemedicine eye screening of neonates has also grown in recent years. Darius Moshfeghi, MD, is leading the Newborn Eye Screen Testing (NEST) study to evaluate the role of universal eye screening in a U.S. population.21
Role of Anti-VEGF Agents
Some pediatric retina specialists are increasingly advocating for the use of anti-VEGF therapy for select cases of ROP, especially those with posterior disease, as well as those with media opacity or poor dilation. While this represents an important addition to our arsenal of available therapies, a number of questions remain.
The Bevacizumab Eliminates the Angiogenic Threat for ROP (BEAT-ROP) study demonstrated the efficacy of bevacizumab (Avastin, Genentech) vs. laser for treatment of zone I, stage 3 with plus disease or posterior zone II, stage 3 with plus disease.22 Anti-VEGF agents are attractive for ROP because they may limit visual field loss from laser, may work faster and may allow the posterior retinal and foveal avascular zone to develop in young children. Anti-VEGF drugs can be administered through media opacity and do not require general anesthesia. Additionally, patients with anti-VEGF therapy may develop less refractive error than those treated with laser.23
However, the systemic effects of anti-VEGF agents on the development of neonates are still unclear. The risk to the developing brain may be particularly high due to impaired blood-brain barrier function. Local adverse events include the risk of reactivation and even “ROP crunch” due to rapid regression and contraction of fibrovascular membranes. The rate and time to recurrence of ROP may be less predictable with anti-VEGF agents than with laser, and late recurrences after anti-VEGF therapy have been reported.24
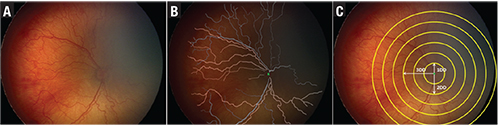 |
| Figure 2. The i-ROP computer-based image analysis system has demonstrated efficacy in diagnosis of plus vs. no plus disease and plus vs. pre-plus vs. no plus disease from fundus photos (A) through an algorithm that calculates acceleration of all vessels (B) in a circular cropped image with radius of 6 disc diameters (C). |
The ideal timing of the injection as well as the ideal anti-VEGF agent and dosing also remain unclear. Most studies of anti-VEGF agents for ROP have used bevacizumab, although limited recent studies have also described use of ranibizumab (Lucentis, Genentech), which has a shorter half-life and potentially fewer systemic effects.27 A recent small retrospective series reported similar outcomes with both agents,28 although another reported a high recurrence rate (83 percent) with ranibizumab.29 Controversy also remains over whether intravitreal anti-VEGF monotherapy, laser monotherapy or combination laser and intravitreal anti-VEGF therapy is best.30
The Phase III RAINBOW study (Ranibizumab compared with laser therapy for the treatment of Infants Born Prematurely with Retinopathy of Prematurity) is designed to answer some of these questions. The study will compare ranibizumab (0.1 mg or 0.2 mg) and laser for zone I ROP with plus disease, zone I ROP with stage 3 disease, zone II, stage 3 plus disease, or aggressive posterior ROP.
Ocriplasmin In Pediatric Vitreoretinal Surgery
Recent studies have raised the question of whether ocriplasmin (Jetrea, Thrombogenics) has a role in facilitating posterior vitreous detachment induction during pediatric vitrectomy. A recent study compared intravitreal ocriplasmin vs. sham injection prior to vitrectomy and found no significant differences in adverse events or in efficacy of PVD induction. The investigators noted improved ease of vitrectomy in most ocriplasmin-treated eyes.31 Further studies of ocriplasmin with larger patient numbers and more refined and objective endpoints are necessary. RS
REFERENCES
1. Kang KB, Wessel MM, Tong J, D’Amico DJ, Chan RV. Ultra-widefield imaging for the management of pediatric retinal diseases. J Pediatr Ophthalmol Strabismus. 2013;50:282–288.
2. Tsui I, Franco-Cardenas V, Hubschman JP, Schwartz SD. Pediatric retinal conditions imaged by ultra wide field fluorescein angiography. Ophthalmic Surg Lasers Imaging Retina. 2013;44:59–67.
3. Fung THM, Muqit MMK, Mordant DJ, et al. Noncontact high-resolution ultra-wide-field oral fluorescein angiography in premature infants with retinopathy of prematurity. JAMA Ophthalmol. 2014;132:108-110.
4. Fung THM, Yusuf IH, Xue K, et al. Heidelberg Spectralis ultra-widefield fundus fluorescein angiography in infants. Am J Ophthalmol. 2015;159:78-84.
5. Klufas MA, Patel SN, Ryan MC, et al. Influence of fluorescein angiography on the diagnosis and management of retinopathy of prematurity. Ophthalmology. 2015;122:1601-1608.
6. Patel SN, Klufas MA, Ryan MC, et al. Color fundus photography versus fluorescein angiography in identification of the macular center and zone in retinopathy of prematurity. Am J Ophthalmol. 2015;159:950-957.
7. John VJ, McClintic JI, Hess DJ, Berrocal AM. Retinopathy of prematurity versus familial exudative vitreoretinopathy: report on clinical and angiographic findings. Ophthalmic Surg Lasers Imaging Retina. 2016;47:14-19.
8. Kashani AH, Brown KT, Chang E, Dresner KA, Capone A. Trese MT. Diversity of retinal vascular anomalies in patients with familial exudative vitreoretinopathy. Ophthalmology. 2014;121:2220-2227.
9. Kashani AH, Learned D, Nudleman E, Dresner KA, Capone A. Trese MT. High prevalence of peripheral retinal vascular anomalies in family members of patients with familial exudative vitreoretinopathy. Ophthalmology. 2014;121:262-268.
10. Mills MD. Retinopathy of prematurity malpractice claims. Arch Ophthalmol. 2009;127: 803-804.
11. Murakami Y, Jain A, Silva RA, et al. Stanford University Network for Diagnosis of Retinopathy of Prematurity (SUNDROP): 12 month experience with telemedicine screening. Br J Ophthalmol. 2008;92:1456-1460.
12. Murakami Y, Silva RA, Jain A, et al. Stanford University Network for Diagnosis of Retinopathy of Prematurity (SUNDROP): 23-month experience with telemedicine screening. Acta Ophthalmol. 2010;88:317-322.
13. Quinn GE, Ying GS, Daniel E, et al; e-ROP Cooperative Group. Validity of a telemedicine system for the evaluation of acute-phase retinopathy of prematurity. JAMA Ophthalmol. 2014;132:1178-1184
14. Fierson WM, Capone A, et al. Telemedicine for evaluation of retinopathy of prematurity: Joint technical report. Pediatrics. 2015;135:e238-e254.
15. Ryan MC, Ostmo S, Jonas K, et al. Development and evaluation of reference standards for image-based telemedicine diagnosis and clinical research studies in ophthalmology. AMIA Annu Symp Proc. 2014;2014:1902-1910.
16. Ataer-Cansizoglu E, Bolon-Canedo V, Campbell JP, et al. Computer-based image analysis for plus disease diagnosis in retinopathy of prematurity: performance of the “i-ROP” system and image features associated with expert diagnosis. Transl Vis Sci Technol. 2015;4:5.
17. Chan RV, Patel SN, Ryan MC, et al. The Global Education Network for Retinopathy of Prematurity (Gen-ROP): Development, implementation, and evaluation of a novel tele-education system (An American Ophthalmological Society Thesis). Trans Am Ophthalmol Soc. 2015;113:T21-T226.
18. Chiang MF, Jiang L, Gelman R, et al. Interexpert agreement of plus disease diagnosis in retinopathy of prematurity. Arch Ophthalmology. 2007;125:875-880.
19. Cabrera MT, Freedman SF, Kiely AE, et al. Combining ROPtool measurements of vascular tortuosity and width to quantify plus disease in retinopathy of prematurity. J AAPOS. 2011;15:40-44.
20. Wittenberg LA, Jonsson NJ, Chan RVP, et al. Computer-based image analysis for plus disease diagnosis in retinopathy of prematurity. J AAPOS 2012; 49(1): 11-20.
21. Callaway NF, Ludwig CA, Blumenkranz MS, Jones JM, Fredrick DR, Moshfeghi DM. Retinal and optic nerve hemorrhages in the newborn infant: One-year results of the Newborn Eye Screen Test Study. Ophthalmology. 2016 Feb 11. [Epub ahead of print]
22. Mintz-Hittner HA, Kennedy KA, Chaung et al. Efficacy of intravitreal bevacizumab for stage 3+ retinopathy of prematurity. NEJM. 2011;364:603-615.
23. Geloneck MM, Chuang AZ, Clark WL, et al. Refractive outcomes following bevacizumab monotherapy compared with conventional laser treatment: a randomized clinical trial. JAMA Ophthalmol. 2014;132:1327–1333.
24. Ittiara S, Blair MP, Shapiro MJ, Lichtenstein SJ. Exudative retinopathy and detachment: a late reactivation of retinopathy of prematurity after intravitreal bevacizumab. J AAPOS. 2013;17:323–325.
25. Lepore D, Quinn GE, Molle F, et al. Intravitreal bevacizumab versus laser treatment in type 1 retinopathy of prematurity: report on fluorescein angiographic findings. Ophthalmology 2014;121:2212–2219.
26. Henaine-Berra A, Garcia-Aguirre G, Quiroz-Mercado H, et al. Retinal fluorescein angiographic changes following intravitreal anti-VEGF therapy. J AAPOS. 2014;18:120-123.
27. Avery RL. Bevacizumab (Avastin) for retinopathy of prematurity: wrong dose, wrong drug, or both? J AAPOS 2012;16:2–4.
28. Chen SN, Lian I, Hwang YC, et al. Intravitreal antivascular endothelial growth factor treatment for retinopathy of prematurity: comparison between ranibizumab and bevacizumab. Retina. 2014;35:667-674.
29. Wong RK, Hubschman S, Tsui I. Reactivation of retinopathy of prematurity after ranibizumab treatment. Retina. 2015;35:675-680.
30. Kim J, Kim SJ, Chang YS, Park WS. Combined intravitreal bevacizumab injection and zone I sparing laser photocoagulation in patients with zone I retinopathy of prematurity. Retina. 2014;34:77–82.
31. Drenser K, Girach Z, Capone A. A randomized, placebo-controlled study of intravitreal ocriplasmin in pediatric patients scheduled for vitrectomy. Retina. 2016;36:565-575.
32. De Paula Freitas B, de Oliveira Dias, JR, Prazeres J, et al. Ocular findings in infants with microcephaly associated with presumed Zika virus congenital infection in Salvador, Brazil. JAMA Ophthalmol. 2016 Feb 9. [Epub ahead of print]



