Optical coherence tomography is an indispensable tool for the diagnosis and management of patients with uveitis. Use of OCT in uveitis is most often directed at monitoring for retinal complications such as cystoid macular edema, epiretinal membranes, subretinal fluid, secondary choroidal neovascularization and inner/outer retinal and retinal pigment epithelium disruption. There are, however, a few more seldom-recognized ways to use OCT in the uveitic patient. I’ll review them here.
 |
Grading AC inflammation
Multiple studies have shown the potential for the use of anterior segment OCT in the grading of anterior chamber inflammation.1,2 In a study by Sumit Sharma, MD, and colleagues, the number of cells seen on AS-OCT line and volume scans strongly correlated with the number of cells seen on clinical examination.1
A later study by Alessandro Invernizzi, MD, and colleagues additionally used swept-source OCT for the grading of anterior chamber flare.2 They used AS-OCT images to calculate an absolute value of aqueous signal intensity, comparing that value to the signal measured outside the eye to arrive at an aqueous-to-air relative intensity index. The researchers found that the ARI index rose with increasing clinical flare measurements and correlated with laser flare photometry measurements in patients with active uveitis.
 |
Evaluation of the vitreous
Evaluation of vitreous cells relies on slit-lamp examination and only looks for inflammation in the anterior vitreous. Because anterior uveitis can result in spillover involvement of the anterior vitreous, the presence of anterior vitreous cells alone does not typically signify a vitritis. Thus, in the absence of visible snowballs or snowbanks, the presence of an overt vitritis can be called into question.
Additionally, the presence of anterior media opacities can make evaluation of the anterior vitreous challenging and confound the grading of vitreous haze. OCT allows routine examination of the posterior cortical vitreous, in which posterior vitreous cells appear as hyper-reflective spots.
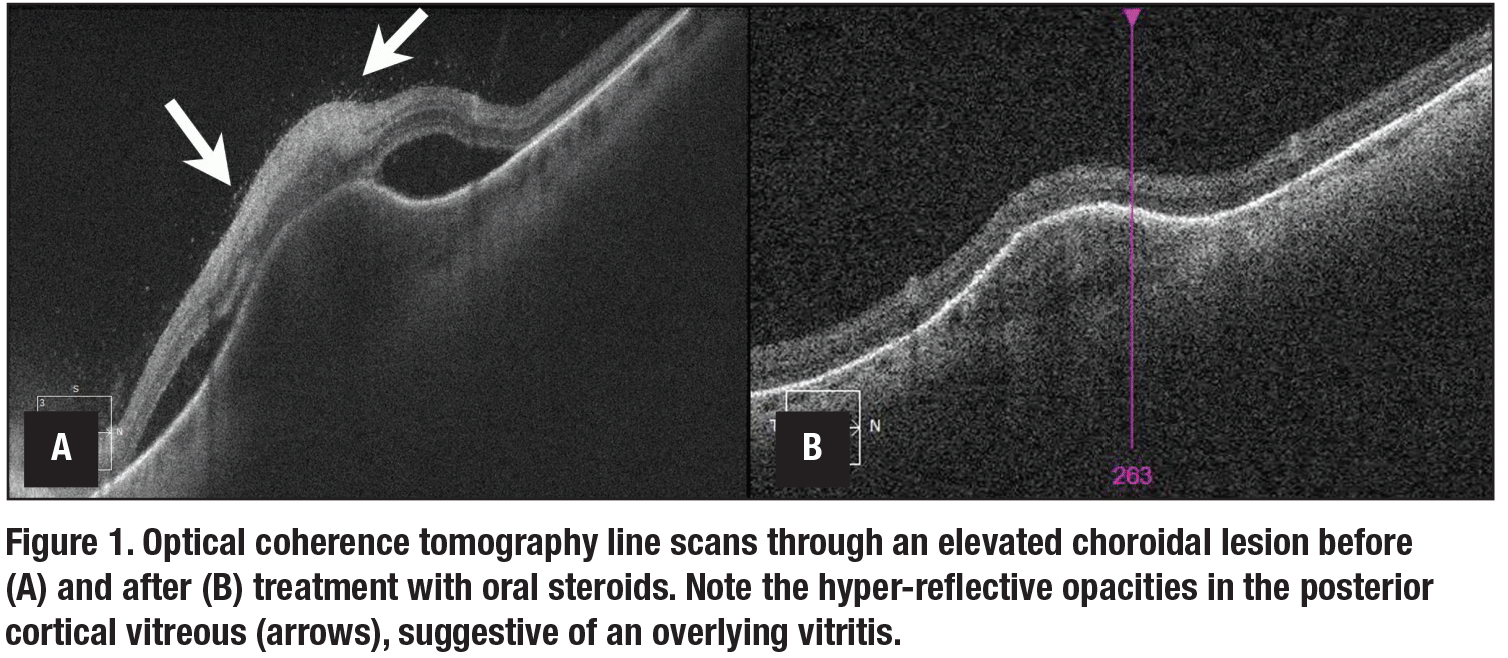
Evaluation of cells in the posterior vitreous can provide important diagnostic information. For example, in the case of an elevated choroidal lesion, presence of overlying vitreous hyper-reflective spots on OCT could signify an inflammatory rather than malignant lesion (Figure 1). Additionally, improvement in such a posterior vitreous cell count can be used to monitor response to therapy.
While other vitreous opacities, such as vitreous hemorrhage, may be indistinguishable on OCT, in vitro data suggest that cell reflectance for various cell types (neutrophils, monocytes, lymphocytes, erythrocytes) may in fact be different.3 In addition to evaluating the posterior vitreous, OCT may be useful for evaluation of anterior vitreous cell and vitreous haze. A clinical trial is currently recruiting patients to evaluate this very question.4
Evaluation of the retinal vasculature
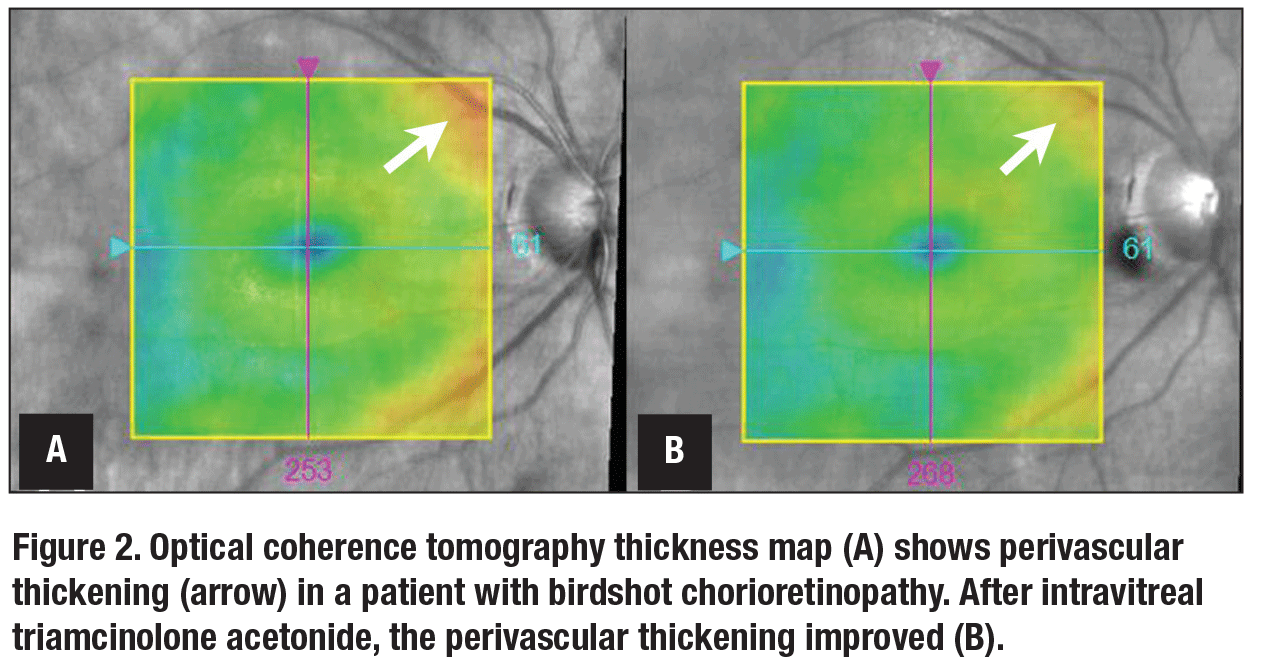
Determining the presence of retinal vasculitis is largely predicated on ophthalmoscopy and fluorescein angiography. However, evaluation of OCT thickness maps can be a valuable adjunct for monitoring retinal vasculitis. A 2018 study reported that perivascular thickening on OCT thickness maps may be a marker of retinal vasculitis in uveitides featuring a large vessel retinal vasculitis, such as birdshot chorioretinopathy (Figure 2).5 The same study showed that the severity of such perivascular thickening corresponded to the severity of perivascular leakage on FA, was independent of CME and central macular thickness, and improved in response to therapy.
 |
Evaluation of the choroid
Enhanced-depth imaging (EDI)-OCT enables evaluation of the choroid, which can prove very informative when managing uveitis. Increased choroidal thickness has been noted in eyes with acute anterior uveitis compared to fellow uninvolved eyes6 and eyes of patients without uveitis.7 Additionally, one study noted that choroidal thickness decreased with therapy.6
Increased choroidal thickness has not been noted in all forms of anterior uveitis. One study found that eyes with HLA-B27-associated anterior uveitis showed similar choroidal thickness values to eyes without uveitis.7 Another series found that eyes with Fuchs’ heterochromic iridocyclitis had choroidal thinning relative to fellow uninvolved eyes.8
 |
Choroidal thickening has also been shown to correspond to disease activity in eyes with active uveitis secondary to
Behçet’s disease.9,10 Additionally, a reduction in choroidal thickness was noted among these patients, with therapy corresponding to other clinical measures of inflammatory control.9,10 One study found that the choroidal thickness in BD decreased with greater duration of disease activity, suggesting that prolonged choroiditis results in choroidal thinning.11
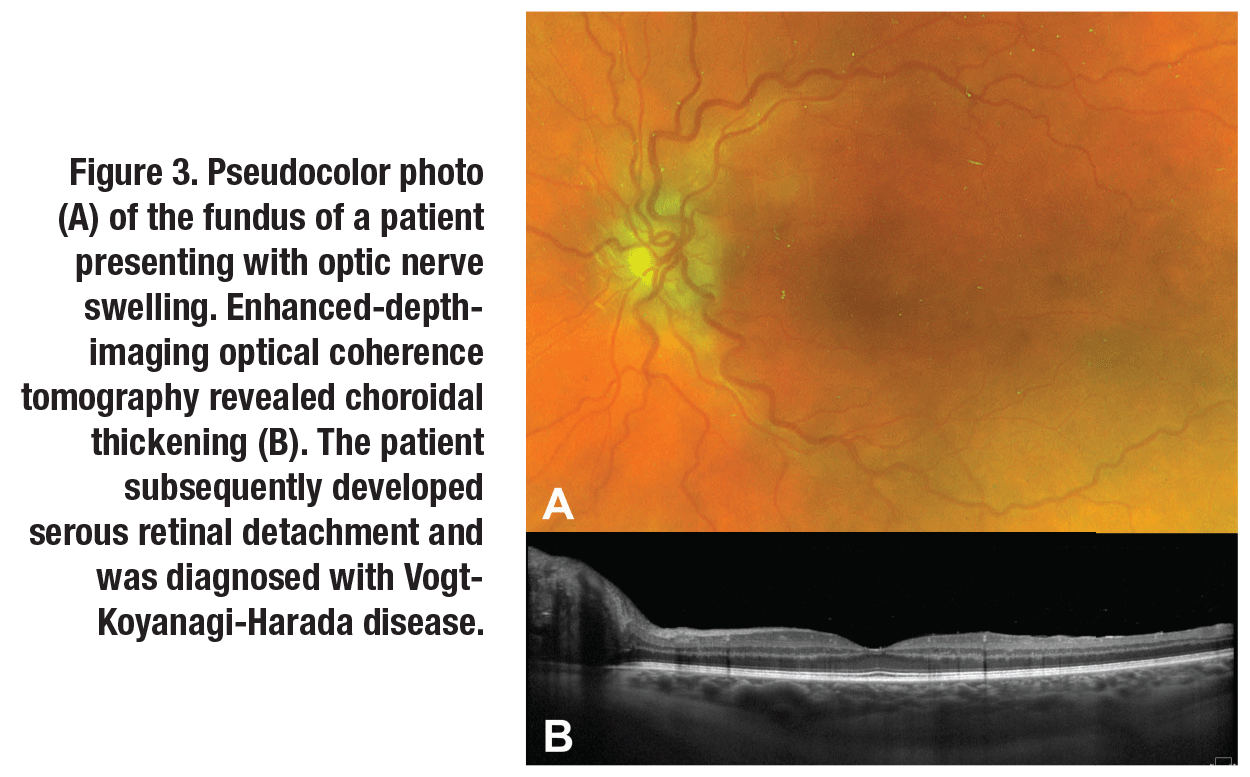
Vogt-Koyanagi-Harada disease can cause choroidal thickening with or without serous retinal detachment. Similar to BD, treatment of inflammation in VKH can cause a corresponding reduction in choroidal thickening (Figure 3, page 16).12,13 Interestingly, the choroidal thickening in VKH seems to correspond to a thickening of the choroidal stroma rather than engorgement of the choroidal vasculature.14
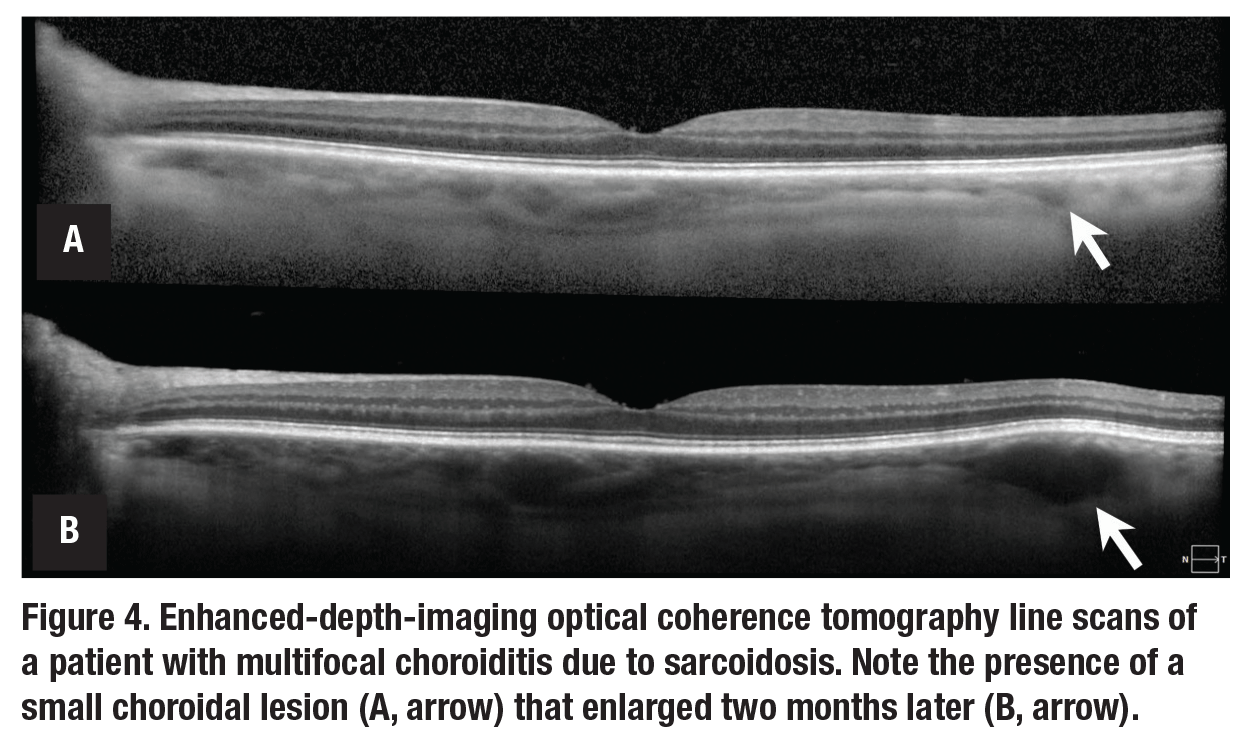
Many uveitides feature a multifocal rather than diffuse choroiditis. In eyes with extensive choroidal lesions, monitoring for the development of new lesions can be cumbersome. Lesions may not be readily evident on ophthalmoscopy, may rely on
indocyanine green angiography and may not always correspond with other markers of disease activity. EDI-OCT can allow for the monitoring of new macular and peripapillary choroidal lesions and the response of such lesions to therapy (Figure 4).15
 |
Bottom line
Overall, potential uses for OCT in the diagnosis and management of uveitis continue to expand, although several of the aforementioned expanded clinical applications of OCT rely on manual measurements (choroidal evaluation), automated algorithms that aren’t widely available (anterior chamber inflammation measurements), semi-quantitative measurements (perivascular thickening analysis) and evaluations for which longitudinal changes may be challenging (posterior vitreous cell measurements).
Given the heterogeneity of uveitis, no single imaging modality can adequately quantify the degree of inflammation across all of its forms. OCT, however, has the potential to measure inflammation and inflammatory sequelae in ocular structures beyond the retina. RS
 |
References
1. Sharma S, Lowder CY, Vasanji A, Baynes K, Kaiser PK, Srivastava SK. Automated analysis of anterior chamber inflammation by spectral-domain optical coherence tomography. Ophthalmology. 2015;122:1464-1470.
2. Invernizzi A, Marchi S, Aldigeri R, et al. Objective quantification of anterior chamber inflammation: Measuring cells and flare by anterior segment optical coherence tomography. Ophthalmology. 2017;124:1670-1677.
3. Rose-Nussbaumer J, Li Y, Lin P, et al. Aqueous cell differentiation in anterior uveitis using Fourier-domain optical coherence tomography. Invest Ophthalmol Vis Sci. 2015;56:1430-1436.
4. Optical coherence tomography (OCT) in uveitis. ClinicalTrials.gov identifier NCT02026128. https://clinicaltrials.gov/ct2/show/NCT02026128?term=NCT02026128&rank=1. Accessed May 15, 2019.
5. Thomas AS, Hatef AL, Stinnett SS, Keenan RT, Jaffe GJ. Perivascular thickening on optical coherence tomography as a marker of inflammation in birdshot retinochoroiditis. Retina. 2019;39:956-963.
6. Gabriel M, Kruger R, Shams-mafi F, et al. Mapping retinal and choroidal thickness in unilateral nongranulomatous acute anterior uveitis using three-dimensional 1060-nm optical coherence tomography. Invest Ophthalmol Vis Sci. 2017;58:4778-4783.
7. Kim M, Choi SY, Park YH. Analysis of choroidal and central foveal thicknesses in acute anterior uveitis by enhanced-depth imaging optical coherence tomography. BMC Ophthalmol. 2017;17:225.
8. Cerquaglia A, Iaccheri B, Fiore T, et al. Full-thickness choroidal thinning as a feature of Fuchs uveitis syndrome: Quantitative evaluation of the choroid by enhanced depth imaging optical coherence tomography in a cohort of consecutive patients. Graefes Arch Clin Exp Ophthalmol. 2016;254:2025-2031.
9. Ishikawa S, Taguchi M, Muraoka T, Sakurai Y, Kanda T, Takeuchi M. Changes in subfoveal choroidal thickness associated with uveitis activity in patients with Behçet’s disease. Br J Ophthalmol. 2014;98:1508-1513.
10. Kim M, Kim H, Kwon HJ, Kim SS, Koh HJ, Lee SC. Choroidal thickness in Behcet’s uveitis: An enhanced depth imaging-optical coherence tomography and its association with angiographic changes. Invest Ophthalmol Vis Sci. 2013;54:6033-639.
11. Park UC, Cho IH, Moon SW, Yu HG. Long-term change of subfoveal choroidal thickness in Behçet’s disease patients with posterior uveitis. Ocul Immunol Inflamm. 2018;26:397-405.
12. Kawano H, Sonoda S, Yamashita T, Maruko I, Iida T, Sakamoto T. Relative changes in luminal and stromal areas of choroid determined by binarization of EDI-OCT images in eyes with Vogt-Koyanagi-Harada disease after treatment. Graefes Arch Clin Exp Ophthalmol. 2016;254:421-426.
13. Jap A, Chee SP. The role of enhanced depth imaging optical coherence tomography in chronic Vogt-Koyanagi-Harada disease. Br J Ophthalmol. 2017;101:186-189.
14. Li M, Liu Q, Luo Y, et al. Enhanced depth SD-OCT images reveal characteristic choroidal changes in patients with Vogt-Koyanagi-Harada disease. Ophthalmic Surg Lasers Imaging Retina. 2016;47:1004-1012.
15. Böni C, Thorne JE, Spaide RF, et al. Choroidal findings in eyes with birdshot chorioretinitis using enhanced-depth optical coherence tomography. Invest Ophthalmol Vis Sci. 2016;57:591-559.



