 |
 |
The annual meeting of the American Society of Retina Specialists in Chicago showcased the latest in diagnostics, treatment and management strategies for retinal disease. The meeting once again demonstrated how rapidly the field of retina is advancing.
Here, we present summaries of five of the most intriguing presentations, primarily related to neovascular age-related macular degeneration. They include an analysis of the relationship between residual fluid on optical coherence tomography and vision outcomes, the effects of fluctuations in retinal thickness on vision outcomes, an investigative oral therapy for nAMD, the increased risk of silicone oil release after agitation of syringes used for intravitreal injections and the safety of abicipar pegol following a modified manufacturing process.
Intraretinal and subretinal fluid and associated vision outcomes
This year’s meeting highlighted the ongoing debate regarding the need for aggressive treatment of fluid on OCT in patients with nAMD. Nancy Holekamp, MD, aimed to provide better understanding of the impact of residual fluid on vision outcomes by performing a retrospective review of 917 treatment-naïve nAMD patients from the HARBOR trial. All patients were treated with ranibizumab (Lucentis, Roche/Genentech) on a monthly or as-needed basis.1
In this cohort, fewer than 30 percent of eyes had residual subretinal (SRF) or intraretinal fluid (IRF) after 12 and 24 months of treatment. In the SRF group, the mean residual SRF thickness was 90.6 μm at 12 months and 82.6 μm at 24 months. Interestingly, eyes with residual SRF had significantly greater improvements in best-corrected visual acuity from baseline than eyes with resolved SRF. The odds of attaining 20/40 or better BCVA were similar for eyes with or without SRF.
Eyes with residual IRF had significantly less improvement in BCVA compared to eyes with resolved IRF. The odds of attaining 20/40 or better BCVA were lower for eyes with residual IRF when compared to eyes with resolved IRF.
Eyes with residual SRF (no IRF) only experienced the greatest gain in BCVA and achieved the highest BCVA letter scores at 12 and 24 months. Vision outcomes were similar among eyes with resolved or residual SRF and IRF. Eyes with only residual IRF (no SRF) had the worst outcomes.
“It’s the treating, not the drying, that’s needed for good vision outcomes,” Dr. Holekamp concluded.
The take home: This study brings into question the need to completely dry all SRF on OCT in nAMD patients. A small amount of SRF on OCT with active anti-VEGF treatment appears to be optimal for long-term vision improvement.
Dr. Holekamp disclosed relationships with Allergan, Genentech, Novartis and Regeneron Pharmaceuticals.
 |
HAWK, HARRIER: CST fluctuations lead to worse visual outcomes
The HAWK and HARRIER Phase III randomized clinical trials compared brolucizumab 3 or 6 mg (Novartis) to aflibercept 2 mg (Eylea, Regeneron Pharmaceuticals) therapy for nAMD. All patients received three monthly loading doses for three months. Brolucizumab patients were then given treatment every 12 weeks, and the treatment interval could be reduced to every eight weeks based upon disease activity. Aflibercept patients were treated every eight weeks after the three loading doses.
This analysis divided HAWK and HARRIER patients into quartiles based upon the standard deviation of each eye’s central subfield thickness (CST) from baseline to week 96.2 The standard deviation was to be used as a metric of individual CST variability. The 444-patient quartiles were divided by SD as follows:
<27 μm (no fluctuation in CST after completion of loading phase).
27-44 μm (dry after loading phase with minor fluctuations in CST).
44-68 μm (moderate fluctuations in CST).
68 μm (marked fluctuations in CST).
 |
Using the first quartile (minimal CST fluctuation) as the reference, the study found that increasing variability in CST was associated with worse BCVA outcomes at week 96. Mean BCVA change at 96 weeks was 10.3 letters for quartile 1, 8.8 letters for quartile 2, 6.9 letters for quartile 3, and 2.1 letters for quartile 4. The latter quartile had a mean gain of 8 letters less than quartile 1.
The study concluded that maintaining stable CST translates into better disease control and superior visual outcomes in nAMD. Furthermore, a more stable CST was associated with a dry retina.
The take home: Based upon this data, retina specialists should prioritize the use of treatments that will minimize fluctuations of CST to achieve the best visual outcomes in nAMD.
Presenter Chirag D. Jhaveri, MD, disclosed relationships with the Diabetic Retinopathy Clinical Research Network,
Genentech, Novartis and Regeneron.
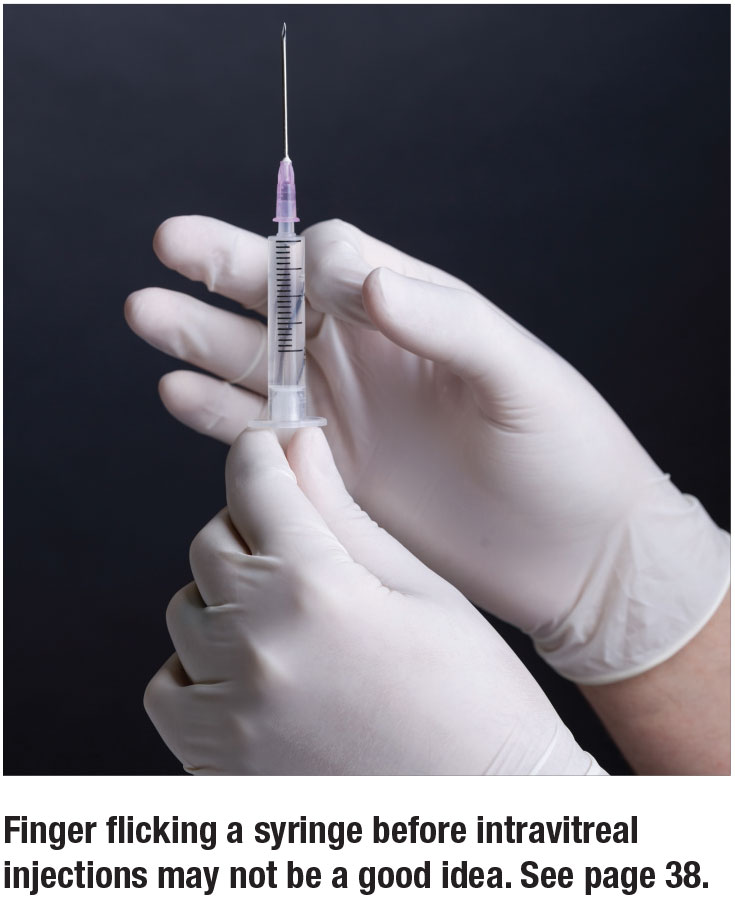
Finger-flicked syringes release SO droplets at higher rates
The 2018 ASRS Practices and Trends survey found that 5.2 percent of retina specialists in the United States have performed a vitrectomy for symptomatic vitreous floaters due to silicone oil (SO) droplets following intravitreal injections of bevacizumab.3 This study assessed the release of SO by eight different models of syringe tested in multiple in vitro conditions. A separate, related study examined the frequency of SO droplets apparent at clinical examination.
Masked graders performed all analyses using light microscopy. The presence of silicone oil was confirmed by Fourier-transform infrared spectroscopy. Of the eight different commonly used syringe models tested, the Braun Injekt-F oil-free syringe was the only one not found to release SO. Of note, with all syringes tested, agitation of the syringe (analogous to “flicking” with one’s finger) led to a statistically significant increase in the amount of SO released.
In the clinical study, 37 eyes undergoing routine intravitreal injections of bevacizumab were compared with 30 control eyes and evaluated with slit-lamp examination and ultrasonography to assess the amount of SO in the vitreous. Slit-lamp examination found SO in the vitreous in 68 percent of treated eyes and ultrasound found SO in 76 percent. The amount of silicone oil was correlated with the number of prior injections.
This study identifies a higher risk of release of SO droplets with most of the currently utilized syringes. The risk increases significantly with agitation.
The take home: We should carefully consider the type of syringe that our compounding pharmacy uses in order to avoid this potential complication, and avoid agitation of the syringe.
Presenter Gustavo Barreto de Melo, MD, PhD, had no relevant financial relationships to disclose.
 |
Oral therapy for nAMD
Not all patients achieve adequate anatomic and/or visual improvements when regularly receiving intravitreal injections of anti-VEGF for nAMD. This study evaluated an oral therapy for nAMD patients refractory to standard anti-VEGF therapy.4
C-C chemokine receptor type 3 (CCR3) and its ligand CCL11 are highly expressed in subretinal neovascular lesions. Inhibition of CCR3 disrupts endothelial cell migration, which reduces the morphological changes associated with pathologic choroidal neovascularization. AKST4290 (Alkahest) is an orally administered small-molecule antagonist of human CCR3.
In this Phase IIa, single-arm, open-
label study, 24 nAMD patients unresponsive to IVT anti-VEGF therapy (i.e., persistent fluid and no BCVA improvement after at least three monthly injections) were given AKST4290 oral monotherapy for six weeks. Seventy-two percent had stable or improved BCVA, and the mean improvement in BCVA was 2 letters. Eight percent of subjects gained ≥10 letters. CST did not change significantly during the six weeks of treatment, but then improved significantly in the subsequent four weeks of follow-up without treatment. The study found AKST4290 to be safe and well-tolerated.
In a related Phase IIa study in treatment-naïve nAMD eyes, BCVA improved in 83 percent of eyes with a mean gain of 7 letters.
The take home: Effective oral therapy for nAMD would be a welcome addition to our treatment armamentarium that could reduce the need for myriad IVT injections and their associated risks and costs.
Presenter Michael W. Stewart, MD, disclosed relationships with Alkahest, Allegan and Regeneron.
MAPLE: Abicipar safety improved following manufacturing changes
Abicipar pegol (Allergan), a DARPin-binding protein, is a pegylated recombinant ankyrin protein that binds all isoforms of VEGF-A with high affinity and specificity. It has been shown in vitro to bind to VEGF-A with a significantly higher affinity than ranibizumab.
The CEDAR and SEQUOIA Phase III trials found abicipar given every eight or 12 weeks at fixed dosing was noninferior to ranibizumab monthly dosing in treatment-naïve nAMD patients. Visual acuity gains were similar across in all three arms of the study. However, these studies noted an increased incidence of intraocular inflammation (IOI) in patients receiving abicipar.
The MAPLE study was designed to evaluate the safety and treatment effect of abicipar manufactured with a modified process to optimize the removal of host-derived impurities.5 The 28-week study included both treatment-naïve nAMD patients and those who had previous anti-VEGF treatments. Three loading doses followed by two doses eight weeks apart were administered in 123 patients.
The overall incidence of IOI was 8.9 percent, down from 13.1 percent and 13.8 percent in CEDAR and
SEQUOIA, respectively. Most events were assessed to be mild to moderate. Severe events of IOI were reported in 1.6 percent of patients (vs. 3.4 and 3 percent in CEDAR and SEQUOIA), all of which resolved with topical and/or oral corticosteroids. There were no reported cases of endophthalmitis or retinal vasculitis.
The take home: CEDAR and SEQUIOA demonstrated the potential of abicipar to reduce treatment burden in nAMD. However, many were alarmed by the elevated IOI rates. The MAPLE study helps to alleviate those concerns by demonstrating an improved safety profile after a modified manufacturing process. The Food and Drug Administration recently accepted a biologics license application for abicipar, bringing us one step closer to a promising new treatment option for nAMD. RS
Presenter Raj K. Maturi, MD, disclosed relationships with Aerpio, Allegro, Allergan, Genentech and Graybug Vision.
REFERENCES
1. Holekamp NM, Ghanekar AA, Hill L, Yang M, Gune S. What do the HARBOR data reveal about the relationship between the drying of subretinal and intraretinal fluid and associated vision outcomes? Presented at American Society of Retina Specialists 37th annual scientific meeting; July 27, 2019; Chicago, IL.
2. Jhaveri CD, Dugel PU, Wykoff CC, et al. 96-week visual and expanded anatomical outcomes of brolucizumab versus aflibercept in patients with nAMD: Results from HAWK and HARRIER. Presented at ASRS 37th annual scientific meeting; July 27, 2019; Chicago, IL.
3. De Melo GB, Emerson GG, Dias Jr. C, et al. Silicone droplets are released by syringes and found in the vitreous. Presented at ASRS 37th annual scientific meeting; July 27, 2019; Chicago, IL.
4. Stewart MW, Jeffords E, Klutzaritz V, Rawner E, Newman E. Open-label study evaluating the effects of the CCR3 antagonist ALK4290 in patients with neovascular AMD refractory to anti-VEGF therapy. Presented at ASRS 37th annual scientific meeting; July 27, 2019; Chicago, IL.
5. Maturi RK, Abicipar Phase 2 MAPLE trial supports improved safety for patients with nAMD following a modified manufacturing process. Presented at ASRS 37th annual scientific meeting; July 30, 2019; Chicago, IL.



