 |
Insulin
There are three basic types of insulins: long-acting; short-acting; and premixed, which combines short- and long-acting insulins. Long-acting insulin is taken often at night, while short-acting insulins are used to deal with meals. Premixed insulin aims to simplify dosing by having both types of insulin in one shot.
For a surgeon, the greatest worry with insulin is the risk of hypoglycemia. For example, if the diabetes patient ordered to take nothing by mouth for surgery takes the usual insulin dose, then the glucose can become too low and he or she may even pass out. Some patients do the opposite and stop all of their insulin because they’re not eating anything, so they think they don’t need insulin. In these cases, they will not have insulin to move glucose into the cell for energy production, which can trigger diabetic ketoacidosis (DKA).
Perhaps the best strategy is to have patients get instructions from their endocrinologist on the proper dosing of insulin for surgery. Also, make sure the dosing is documented; hopefully, this will minimize the risk of both hypoglycemia and DKA.
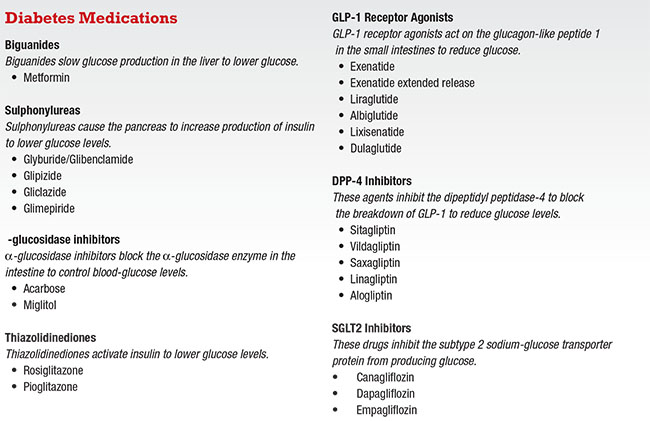
Biguanide (Metformin)
Normally, the liver supplies glucose when we are not eating; that keeps us alive as we sleep. But in diabetes, the liver produces too much glucose. Biguanides work by slowing down this process, thereby lowering glucose. Metformin is the only available biguanide.
Metformin does not cause hypoglycemia, but at higher dosages it can cause diarrhea. Metformin is also cleared through the kidneys. If renal function declines, then metformin can accumulate, and this could lead to lactic acidosis. So if the estimated glomerular filtration rate (eGFR) is less than 60, then the patient needs to reduce the metformin dosage; and if the eGFR goes below 30, the patient should stop metformin.
The concern for surgeons with patients on metformin is dehydration perioperatively. The dehydration could worsen renal function enough that the metformin accumulates, which could then lead to lactic acidosis. Contrast dyes for imaging can also worsen renal function. Radiology will often ask for the creatinine and eGFR levels before using contrast dyes in patients with diabetes. So for elderly patients on metformin, it is important to have a recent creatinine and eGFR on file for reference.
Sulphonylureas
There are several molecules in the sulphonylurea category: glyburide/glibenclamide, glipizide and glimepiride. Sulphonylureas make the beta cells in the pancreas secrete more insulin, which then lowers glucose levels. They are inexpensive, and the glucose drop is rapid and satisfying.
 |
Unfortunately, these medications continue to push out insulin even when glucose levels are low. Clinical trials have shown hypoglycemia occurring in up to 40 percent of patients using these medications. Also, because the pancreas works so hard, eventually it loses its ability to secrete insulin, requiring additional medications.
Sulphonylureas may increase the risk of hypoglycemia when patients are NPO before surgery, so their glucose levels need to be monitored. Also, sulphonylureas are cleared through the kidneys, so dehydration and worsening of renal function could lead to an accumulation of these medications, which could also cause hypoglycemia.
α-glucosidase Inhibitors
Acarbose and miglitol block the α-glucosidase enzyme in the intestine, which normally breaks down starch into individual glucose molecules so that the gut can absorb them. Without that breakdown, the body could not absorb glucose. Bacteria in the colon eventually processes undigested starches, which unfortunately produces gas, hence the main side effect of this class of medication—flatulence.
α-glucosidase inhibitors do not cause hypoglycemia on their own, but they are often used with other medications that can cause hypoglycemia.
The key issue, though, with this class of medication is that when patients taking them become hypoglycemic, the usual treatments do not work. For example, normal table sugar will not work because the enzyme is blocked and they cannot break down the sugar into single molecules.
These patients would need pure glucose tablets instead to treat the hypoglycemia. So for patients on alpha-glucosidase inhibitors, glucose tablets should be available in the office to treat their hypoglycemia appropriately.
 |
Thiazolidinediones
Thiazolidinediones (TZDs) include pioglitazone and rosiglitazone. As insulin sensitizers, TZDs make insulin work better.
Rosiglitazone was implicated in causing myocardial infarctions and death, although this was later dispelled.
TZDs can cause weight gain and edema, and have been associated with heart failure and bone fractures. They do not cause hypoglycemia on their own.
GLP-1 Receptor Agonists
GLP-1, which stands for glucagon-like peptide, is a hormone the small intestine releases when we eat. The bloodstream transports GLP-1 to the pancreas and signals the beta cells to produce insulin because food is on the way. GLP-1 also signals alpha cells in the pancreas to stop making glucagon, the hormone that puts glucose into the bloodstream when we are not eating. Obviously with food coming in, there is no need for glucagon production. So when you eat, GLP-1 turns on insulin and turns off glucagon.
However, in patients with type 2 diabetes, GLP-1 does not activate fast enough after meals, and they also do not make enough GLP-1 compared to patients without diabetes. This GLP-1 deficiency means that insulin levels do not increase properly and the glucagon does not decrease properly. This combination results in higher glucose levels.
The discovery of reduced GLP-1 in patients with type 2 diabetes led to the concept of restoring GLP-1 back toward normal levels. But GLP-1 is a large-protein hormone so it has to be injected into the body. In normal humans, the DPP-4 enzyme (for dipeptidyl peptidase) inactivates GLP-1 in two minutes. Hence, scientists looked for analogs of GLP-1 that appeared similar, but were different enough that they would not break down so fast.
Exenitide was found in the saliva of the gila monster lizard.2 It has about 50 percent of the same amino acid sequence as human GLP-1, so it does not break down too quickly and can be given twice a day. Liraglutide was created by adding amino acids to human GLP-1,3 and it can be given once a day. Once-weekly formulations are now available as well.
These agents do not cause hypoglycemia, but they do cause nausea, especially when patients first take them as they titrate the medicine upward. This is important to know after eye surgery because nausea and vomiting may increase intraocular pressures. Ideally, patients should not start these GLP-1 agents pre- or postoperatively. An association with pancreatitis has been reported, so patients with a history of pancreatitis should not take them.
DPP-4 Inhibitors
DPP-4 normally breaks down GLP-1, but levels of GLP-1 are already too low in patients with type 2 diabetes. Hence, a DPP-4 inhibitor slows the breakdown of GLP-1, allowing for more GLP-1 to be present and to do its job properly.
DPP-4 inhibitors are not large proteins, so patients can take them orally as a pill. They do not cause hypoglycemia and are cleared through the kidneys, so dosing needs to be lowered with lower eGFRs.
DPP-4 inhibitors were initially associated with pancreatitis, so avoiding them in patients with a history of pancreatitis is recommended. Many of these DPP-4 inhibitors have been combined with metformin into a single tablet. This means that all the precautions that were mentioned about metformin would apply to these DPP-4 inhibitors/metformin combination therapies as well.
SGLT2 Inhibitors
Glucose is a small molecule, so in the kidneys it will leak out into the urine. But glucose is the body’s fuel source and SGLT (sodium-glucose transporter protein) acts as a pump that pulls the glucose from the urine back into the bloodstream after it filters out. In patients with diabetes, a large amount of glucose ends up in the urine. SGLT senses all this wasted energy and works hard to pull it back. This overactive SGLT pump, unfortunately, keeps the blood glucose levels very high.
So one way to lower glucose would be to block these SGLT pumps and let the glucose leave the body in the urine. The SGLT1 pumps about 10 percent of the glucose back, while SGLT2 pumps handle about 90 percent. So SGLT2 inhibitors were made to give maximum blocking of the glucose pump.
SGLT2 inhibitors do not cause hypoglycemia, but they do cause water and salt loss along with glucose into the urine, so they are like mild diuretics. This means that at the time of surgery, patients can get more dehydrated from these medications. DKA has been reported in patients using insulin with SGLT2 inhibitors when they get dehydrated or stop their insulin.
Call the Endocrinologist
For advice on these medications around the time of surgery, consult with patients’ endocrinologists or treating physician about appropriate dosing of insulin and all their other diabetes medications. The typical strategy is to have patients stop or reduce these medications around the time of surgery and then restart them afterward.
Patients with diabetes will become the largest single group any health-care provider sees. The worry with patients who have diabetes is the risk of hypoglycemia and dehydration around the time of surgery. We all need to stay informed about their treatments and any precautions we need to take.
The number of patients with diabetes will continue to grow, as will the number of medications that they take. Our job is to make sure that we get the best out of these medications while we minimize the harm. RS
REFERENCES
1. World Health Organization. Global Report on Diabetes. http://www.who.int/diabetes/global-report/en/ Geneva, Switzerland. Published 2016. Accessed August 30, 2016.
2. Parkes DG, Mace KF, Traugmann ME. Discovery and development of exenatide: the first antidiabetic agent to leverage the multiple benefits of the incretin hormone, GLP-1. Expert Opin Drug Discov. 2013;8:219-244.
3. Rossi MC, Nicolucci A. Liraglutide in type 2 diabetes: from pharmacological development to clinical practice. Acta Biomed. 2009;80:93-101.
Other sources referenced but not cited:
Professional Practice Committee for the Standards of Medical Care in Diabetes—2016. Diabetes Care. 2016; 39 (suppl 1):S107-S108.
Canadian Diabetes Association Clinical Practice Guidelines Expert Committee. Canadian Diabetes Association 2013 Clinical Practice Guidelines for the Prevention and Management of Diabetes in Canada. Can J Diabetes. 2013;37(suppl 1):S1-S212.
Pharmacologic Management of Type 2 Diabetes: 2016 Interim Update. Can J Diabetes. 2016;40:193–195.



