 |
 |
 |
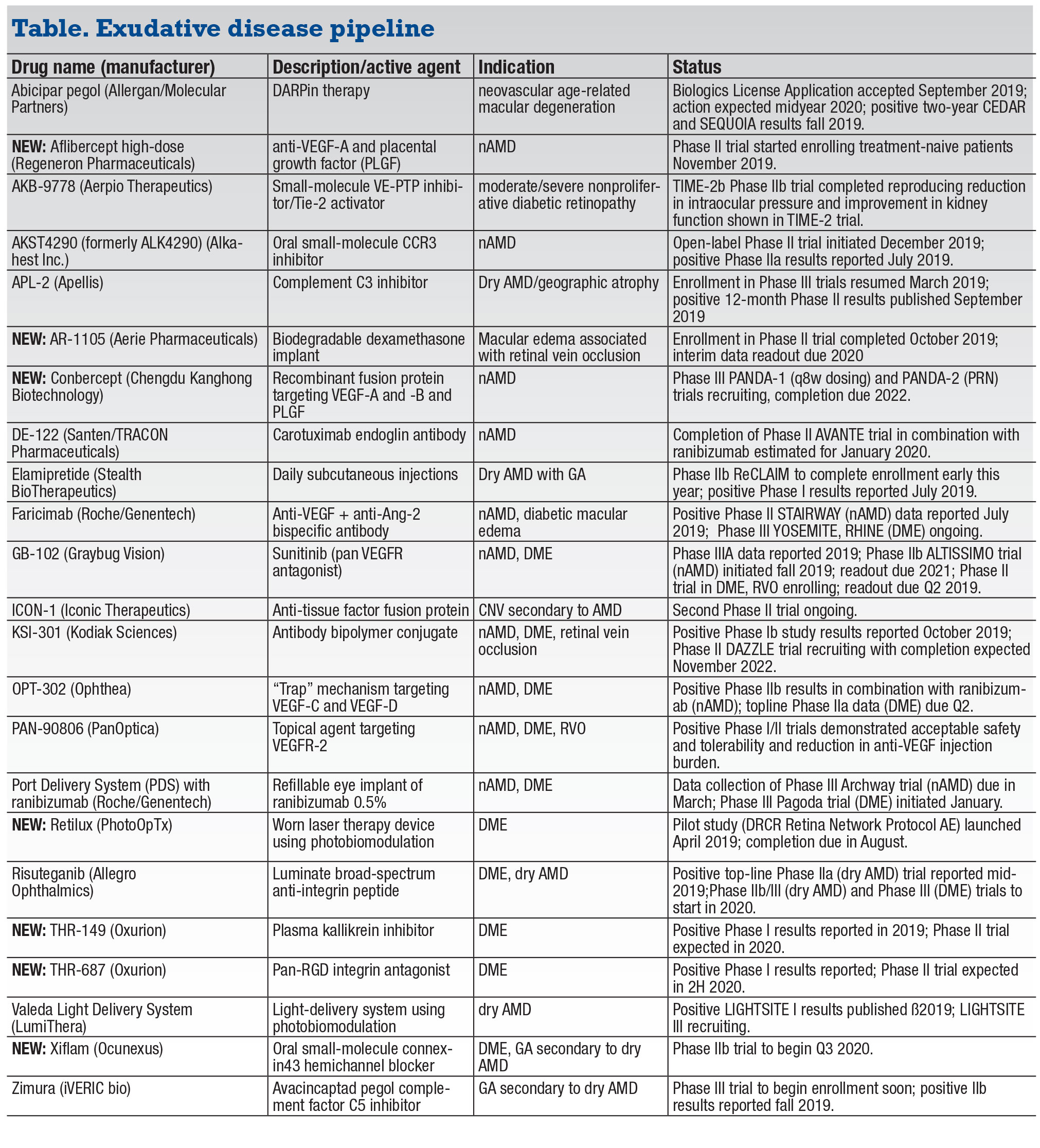
The year ahead promises another robust round of trial readouts and at least one major approval in the arena of biologic and chemical agents to treat exudative disease (and two light-activated therapies). Most notable is the anticipated approval of abicipar pegol, the DARPin therapy for neovascular age-related macular degeneration that Allergan and Molecular Partners are developing. Human trials of no less than 20 other agents (and the aforementioned light-activated therapies) are ongoing, with many expected to provide readouts in 2020.
This year’s list consists of 23 entries, three more than last year. The past year was notable for three major approvals that resulted in exits from our list: Novartis’ Beovu (brolicizumab) for nAMD; and two for Regeneron Pharmaceuticals’ Eylea (aflibercept)—an indication for all stages of diabetic retinopathy and the prefilled syringe.
Another notable exit is THR-317, a placental growth factor antibody. Oxurion pulled investment in the program last year, choosing to focus instead on two other DME agents, both new to this year’s list. Also new this year are high-dose aflibercept; the AR-1105 biodegradable dexamethasone implant (Aerie Pharmaceuticals); conbercept anti-VEGF (Chengdu Kanghong Biotechnology); the light-activated therapy Retilux (PhotoOpTx); and Xiflam (Ocunexus) oral agent.
This listing only includes therapies in human trials or soon to be in the clinic. The narrative omits agents discussed in the previous article on geographic atrophy treatments, although the listing includes them.
Abicipar pegol
Last fall the FDA accepted the Biologics License Application for abicipar pegol. This is usually a precursor to approval, and that could come well before the midyear time frame Allergan and Molecular Partners say they expect.
Encouraging two-year results of the Phase III CEDAR and SEQUOIA trials have shown noninferiority of eight- and 12-week regimens of abicipar 2 mg compared with four-week treatment with Lucentis (ranibizumab, Roche/Genentech).1 Outcomes for changes in best-corrected visual acuity and central retinal thickness were also similar between the three treatment groups.
Reformulation of the drug aimed to address the high rates of intraocular inflammation reported in the first-year CEDAR and SEQUOIA results—over 15 percent in the abicipar arms vs. 0.6 percent in the Lucentis arm. Recent two-year results showed the pooled rate of new cases of intraocular inflammation was 1.9 percent in both abicipar arms vs. 1 percent in the Lucentis arm.
 |
Aflibercept high-dose (Regeneron Pharmaceuticals)
A Phase II trial of in treatment-naïve nAMD started enrolling patients last November. The trial is designed to randomize 100 patients to Eylea (aflibercept 2 mg) or higher-dose and higher-frequency treatments. An earlier previous trial of 33 eyes resistant to other anti-VEGF agents switched them to Eylea every eight weeks, escalated to every four weeks, and then switched to aflibercept 4 mg every four weeks.2 Nine percent of eyes developed geographic atrophy during follow-up, but none had adverse events after the higher-dose therapy.
AKB-9778 (Aerpio Pharmaceuticals)
This first-in-class Tie2 activator binds to and inhibits vascular endothelial protein tyrosine phosphatase (VE-PTP). Patients self-administer the drug subcutaneously. Last year, Aerpio completed the Phase IIb TIME-2b clinical trial in patients with nonproliferative DR. Those results extended out to 48 weeks the reduction in intraocular pressure and improved kidney function in patients with early DR reported in TIME-2.
Three-month TIME-2 results showed that AKB-9778 in combination with Lucentis achieved a 50 percent greater reduction in central subfield thickness and a 58-percent improvement in retinal drying than ranibizumab alone.3 Aerpio is also conducting preclinical studies of ARP-1536, an anti-VE-PTP Tie2 activator that might be a useful adjunct to anti-VEGF therapy in DME.
AKST4290 (Alkahest)
AKST4290 is an oral small-molecule CCR3 inhibitor that targets choroidal blood flow in AMD. Alkahest initiated an open-label Phase II trial, called AKST4290-206 (STEEL), in patients with nAMD in one eye. The Phase IIa trial, known as AKST4290-202, evaluated the agent in nAMD patients no longer responding to anti-VEGF injections.4 Twenty-six patients took AKST4290 400 mg b.i.d. for six weeks; 72 percent showed improvement or maintenance of BCVA.
AR-1105
Aerie is best-known for its glaucoma franchise, having received FDA approval for Rhopressa (netarsudil) and Rocklatan (netarsudil and latanoprost) for lowering IOP. AR-1105 is a sustained release bio-erodible intravitreal dexamethasone implant for macular edema associated with retinal vein occlusion. Last October the company completed enrollment in a Phase II trial for this indication (n=45). The company expects to report interim results on the first 20 patients this year. Aerie has also entered the clinic with AR-13503, a sustained-release implant containing the active metabolite of netarsudil, for nAMD and DME.
 |
Conbercept
Conbercept is an anti-VEGF recombinant fusion protein that was approved in China in 2013. Like Eylea, conbercept targets VEGF-A and -B and PLGF (placental growth factor). Preclinical studies have shown it has a greater affinity for binding to vascular endothelial growth factor than Lucentis.5
Two global Phase III trials in nAMD started recruiting in the past year: PANDA-1 and PANDA-2. Each trial is evaluating 1,140 patients randomized to conbercept, 0.5 or 1 mg, or Eylea 2 mg, with primary efficacy analysis at 36 weeks. PANDA-1 patients will receive three loading doses through week eight, then continue with dosing every eight weeks through week 92. PANDA-2 is a trial of pro re nata dosing after week 40, with the 0.5-mg conbercept and Eylea groups on the same regimen as PANDA-1 up until that point, after which the conbercept 1-mg arm shifts to 12-week dosing after eight weeks and moves onto PRN at week 40. PANDA-1 is due for completion next January and PANDA-2 in March 2022.
DE-122 (carotuximab, Santen Pharmaceutical/Tracon)
The Phase II AVANTE trial in nAMD aims to enroll 76 patients to evaluate DE-122 in combination with ranibizumab vs. ranibizumab alone. The study completion date posted in ClnicalTrials.gov is January 2020.
Faricimab (Roche/Genentech)
This bispecific antibody binds and neutralizes both angiopoietin-2 (Ang-2) and VEGF-A. Twenty-four week Phase II BOULEVARD results showed that treatment-naïve patients receiving faricimab 6 mg for nAMD gained on average 3.6 letters more than patients on Lucentis (p=0.03).6 Other positive faricimab outcomes were a higher percentage of macular leakage resolution in both treatment-naïve and previously treated patients, and a potential for longer time to retreatment compared to Lucentis. The Phase III YOSEMITE and RHINE trials are evaluating extended interval dosing in DME.
GB-102 (sunitinib, Graybug Vision)
This pan-VEGF inhibitor is dosed twice yearly for choroidal neovascularization. Once injected, GB-102 forms a depot of pegylated PLGA microparticles containing sunitinib that biodegrades and releases over time. Six-month Phase I/IIa ADAGIO trial results in nAMD reported that between 50 and 88 percent of patients treated with one dose of GB-102 (either 0.25, 0.5, 1 or 2 mg) were rescue-free after three loading doses of anti-VEGF.7 Last fall the Phase IIb ALTISSIMO trial in nAMD started enrollment, with a readout due next year. A Phase II trial in DME and RVO is also ongoing with a readout due in the second quarter.
ICON-1 (Iconic Therapeutics)
This fusion protein binds to tissue factor overexpressed in the retina and choroid of patients with AMD. A second Phase II study is evaluating intravitreal ICON-1 in combination with Eylea for CNV in AMD. The first Phase II trial, EMERGE, demonstrated biological activity and the ability to target important clinical endpoints. Iconic is also evaluating ICON-4, an anti-tissue factor monoclonal antibody for AMD, with the aim of starting clinical trials this year. Last year Iconic signed an agreement giving Novartis an option on its ophthalmology portfolio.
 |
KS-301 (Kodiak Sciences)
The Phase Ib study of KS-301 demonstrated that about 80 percent of nAMD and DME treated eyes were extended to four months or longer without treatment.8 Treated patients also demonstrated an average 9-letter gain in BCVA and a 121-µm reduction in CST at 90 days. The Phase II DAZZLE study is recruiting about 400 patients with treatment-naïve nAMD. It will compare KS-301 and Eylea.
OPT-302 (Opthea)
Australia-based Opthea describes this drug as a potent inhibitor of VEGF-C and D which binds to VEGF receptors 2 and 3—important distinctions from the other
anti-VEGF drugs with which it has been paired in studies. Last fall, Opthea reported topline data from the Phase IIb trial
of 366 patients with treatment-naïve nAMD treated with both OPT-302 and VEGF-A inhibitor Lucentis or Lucentis alone. The combination group gained 14.2 letters from baseline, on average, vs. 10.8 letters for the Lucentis-only group. Topline data from
the Phase IIa trial in DME of OPT-302 in combination with Eylea, which inhibits both VEGF-A and B, are due in the second quarter.
PAN-90806 (PanOptica)
This once-daily topical drop targets VEGF receptor 2 (VEGFR2). Phase I/II trials in nAMD confirmed safety and tolerability of three doses: 2 mg/mL; 6 mg/mL; or 10 mg/mL. Six percent of patients (n=51) discontinued the drop, but none of the reported adverse events was serious. The trial evaluated PAN-90806 monotherapy in nAMD and proliferative DR without DME over eight weeks as well as maintenance therapy in nAMD following Lucentis injection over 12 weeks. Patients averaged three fewer injections than they would have had on monthly dosing.
Port Delivery System with ranibizumab (Roche/Genentech)
Genentech initiated the Phase III Pagoda study of the PDS in DME of this refillable, surgically implanted mini-reservoir filled with 100 mg/mL of ranibizumab, releasing the drug for up to six months. The Phase III Archway trial of the PDS in nAMD is evaluating 418 patients. Data collection is due to finish in March, with completion next year.
Retilux
This device is worn like an eye patch to deliver laser therapy directly to the effected eye. A DRCR Retina Network Protocol AE trial aims to randomize 134 patients to Retilux or a sham device. Study participants wear the device over the affected eye twice a day for 90 seconds. Enrollment started last spring.
Risuteganib (Luminate, Allegro Ophthalmics)
This synthetic arginyl-glycyl-aspartic acid-class broad-spectrum peptide regulates the functions of several integrin isoforms. Topline results of the first Phase IIa intermediate dry AMD clinical trial showed 48 percent of patients in the risuteganib arm at week 28 gained 8 letters or more from baseline, compared to 7 percent of patients in the sham group at week 12 (p=0.013). The study also confirmed the drug’s safety. Allegro anticipates starting a larger Phase IIb/III U.S. trial in the first half of the year. A Phase III study for DME is also on the drawing board.
 |
THR-149, THR-687 (Oxurion)
Oxurion’s two new VEGF-independent treatments for DME are the plasma kallikrein inhibitor THR-149 and the pan-RGD integrin antagonist THR-687. The Phase I study of THR-149 showed an average improvement of 6.4 letters in BCVA at three months. The Phase I trial of THR-687 showed a BCVA improvement of 8.3 letters at three months. The company expects to move into Phase II for both this year.
Valeda Light Delivery System (LumiThera)
Valeda uses a process called photobiomodulation. The LIGHTSITE III trial last fall enrolled its first patients in a U.S. multicenter study for dry AMD, with the goal to enroll 96 patients. Already approved in Europe, results of the pilot LIGHTSITE I trial reported that about half of treated patients (n=30, 46 eyes) gained at least 5 letters in BCVA vs. 13.6 percent in the sham group (p<0.01) after two series of treatments.9
Xiflam
This oral small-molecule agent targets connexin43 hemichannels that are overexpressed in exudative retinal disease. Xiflam aims to block the opening of these hemichannels at the cell membranes, preventing release of inflammatory mediators. The company expects to start its clinical trial in the third quarter. RS
REFERENCES
1. Khurana R. Abcipar for neovascular AMD: Two-year results from CEDAR and SEQUOIA Phase 3 clinical trials. Paper presented at Retina 2019 Subspecialty Day AAO 2019; San Francisco, CA; October 11, 2019.
2. You QS, Gaber R, Meshi A, et al. High-dose high-frequency aflibercept for recalcitrant neovascular age-related macular degeneration. Retina. 2018;38:1156-1165.
3. Campochiaro PA, Khanani A, Singer M, et al; TIME-2 Study Group. Enhanced benefit in diabetic macular edema from AKB-9778 Tie2 activation combined with vascular endothelial growth factor suppression. Ophthalmology. 2016;123:1722-1730.
4. Stewart MW. Open-label study evaluating the effects of the CCR3 antagonist ALK4290 in patients with neovascular AMD refractory to anti-VEGF therapy. Paper presented at American Society of Retina Specialists 37th annual Scientific Meeting; Chicago, IL; July 27, 2019.
5. Zhang M, Yu D, Yang C, Xia Q, Li W, Liu B, Li H. The pharmacology study of a new recombinant human VEGF receptor-fc fusion protein on experimental choroidal neovascularization. Pharm Res. 2009;26:204-210.
6. Hershberger V. Simultaneous Inhibition of Ang-2 and VEGF-A With Faricimab in DME: Additional Anatomical and Durability Outcomes From the BOULEVARD Phase 2 Trial. Paper presented at American Society of Retina Specialists 37th annual Scientific Meeting; Chicago, IL; July 30, 2019.
7. Liao DS, Grossi FV, El Mehdi D, et al. complement c3 inhibitor pegcetacoplan for geographic atrophy secondary to age-related macular degeneration: A randomized Phase 2 trial. Ophthalmology. 2019 Jul 16. pii: S0161-6420(18)33132-4. [Epub ahead of print]
8. Wykoff C. Extended durability in exudative retinal disease using the novel intravitreal anti-VEGF antibody biopolymer conjugate KSI-301: Results from the Phase IB study in patients with nAME, DME, and RVO. Paper presented at Retina 2019 Subspecialty Day AAO 2019; San Francisco, CA; October 11, 2019.
9. Markowitz SN, Devenyi RG, Munk MR, et al. A double-masked, randomized, sham-controlled, single-center study with photobiomodulation for the treatment of dry age-related macular degeneration. Retina. 2019 Aug 9. [Epub ahead of print]



