 |
 |
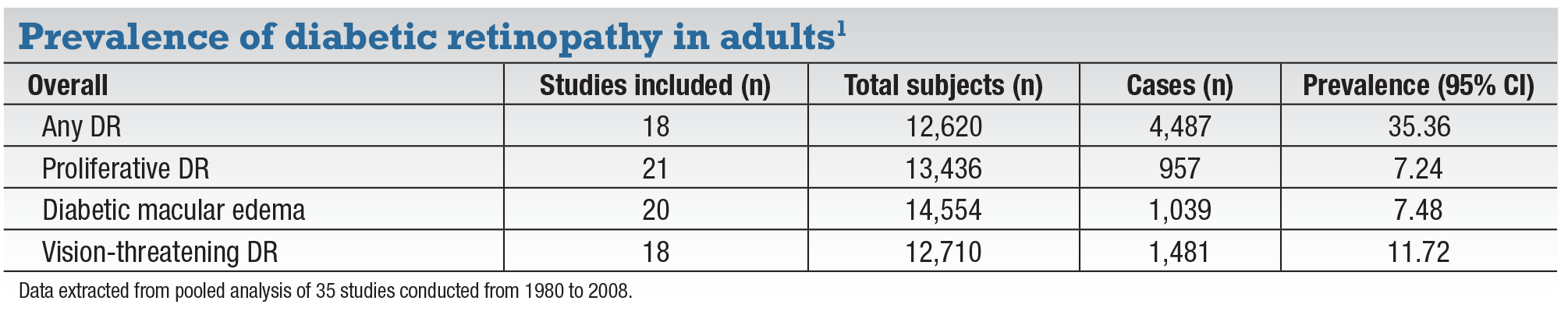
Studies have estimated that one-third of patients with diabetes have diabetic retinopathy, a third of which is vision-threatening; that is, severe nonproliferative DR, proliferative DR or diabetic macular edema. Recent therapeutic advances have improved control, and in some cases prevented development, of DR-related ocular complications.1
Leading that charge have been vascular endothelial growth factor inhibitors. They have revolutionized the management of DME, and multiple landmark studies have demonstrated benefit of treatment with anti-VEGFs.2,3 While anti-VEGF agents have become the standard of care for treatment of DME, timing and frequency of treatments remain variable amongst treating physicians. Nonetheless, emerging data continue to support the importance of early and frequent treatment in eyes with DME.
Evidence from clinical trials
In the pivotal RISE and RIDE studies evaluating ranibizumab (Lucentis, Roche/Genentech) for DME, the sham group was switched to ranibizumab in the third year of follow-up. With this delayed
anti-VEGF treatment, patients in the sham group still gained vision. However, the extent of the visual gains in the sham group (4.7 and 4.3 letters in RIDE and RISE, respectively) were lower than that of patients who had been originally randomized to ranibizumab (10.6 and 14.2 letters, respectively).2
Similarly, in the pivotal VISTA and VIVID trials evaluating aflibercept (Eylea, Regeneron Pharmaceuticals) for DME, patients in the laser group upon switching to aflibercept had significant visual gains. However, their final visual acuity gain from baseline was lower (2.2 and 3.8 letters in VISTA and VIVID, respectively) than the patients originally assigned to aflibercept (9.7 and 13.6 letters, respectively).3
It’s worth noting that patients in the laser group who were switched to aflibercept had to have significant vision loss per protocol criteria. Further study has demonstrated that delayed treatment may have greater negative consequences, with greater risk of subretinal fluid, severe edema, large cysts or renal disease.4
Recently, results from the Diabetic Retinopathy Clinical Research Network-led Protocol V study evaluated three treatment strategies—initial observation, laser or aflibercept therapy—for eyes with center-involved DME and good vision.5 The study concluded that observation until vision worsens is a viable option. Approximately one-third of eyes managed with initial observation required treatment with aflibercept based on vision loss per protocol criteria. Further evaluation of the clinical course of eyes that needed rescue treatment will provide a better understanding of the appropriate timing of intervention.
 |
Real-world findings
Pivotal trials such as RIDE/RISE and VISTA/VIVID required consistent and frequent dosing that resulted in excellent visual outcomes. Emerging real-world data suggest that patients are being under-
treated in clinical practice resulting in sub-optimal outcomes.6,7
 |
In our recent retrospective analysis of the Vestrum Health Database, which included patients from more than 250 retina specialists, more than 40 percent of the patients received fewer than six injections in the first year of treatment resulting in visual gains significantly inferior to those who received more frequent injections during the same period (+3.7 vs. +8 in terms of visual acuity score, respectively).8
Furthermore, for patients who received less frequent treatment in the first year, increasing treatment frequency in the second year didn’t provide additional benefit. Thomas Ciulla, MD, and colleagues also reported a similar relationship of lower visual gains with less frequent treatment based on their analysis of the American Academy of Ophthalmology Intelligent Research in Sight (IRIS) database.9 Interestingly, we also found that the annual trend on injection frequency for DME during the first year of treatment has not shifted in the last few years, highlighting the persistence of undertreatment in clinical practice.
Shift toward earlier intervention
The landmark Early Treatment Diabetic Retinopathy Study (ETDRS) demonstrated that if left untreated, DR progression rates increase based on severity.10 In general, the AAO recommends that eyes with mild or moderate NPDR without clinically significant DME can be observed.11 Panretinal photocoagulation or anti-VEGF therapy is suggested for severe NPDR and PDR. A shift toward earlier intervention for DR is beginning, with repeated evidence that it potentially prevents vision-threatening complications.
PRP has been the most common approach for treatment of DR. The adverse effects as a result of PDR are well-established and are associated with the intensity, area and number of PRP treatments.12 High-risk PDR can require significant PRP. However, PRP administered at an earlier stage—e.g., for severe NPDR—is likely to reduce the amount of overall treatments and, hence, reduce the associated adverse effects.13
 |
Recent data supports anti-VEGF as an excellent alternative for treatment of DR with a better safety profile than PRP. DRCR.net-led Protocol S compared PRP with intravitreal ranibizumab as frequently as every four weeks in eyes with PDR.14 Even though the injection group was noninferior to PRP treatment at two years in terms of visual change, mean peripheral visual field sensitivity loss was worse and vitrectomy and DME formation were more frequent in the laser group. Similarly, in the CLARITY trial, aflibercept was found to be superior to PRP with regards to visual acuity at 52 weeks in a similar population.15
The possibility of achieving DR regression using anti-VEGF therapy offers the opportunity to prevent vision-threatening complications with early intervention. Two-step and three-step improvements in Diabetic Retinopathy Severity Score (DRSS) have been seen in multiple trials. Post-hoc analysis of the RISE and RIDE studies demonstrated greater benefit in patients with baseline moderately severe to severe NPDR with two-step or greater regression in DRSS in 78 percent of patients vs. 31 percent in those with baseline PDR.16
Most recently, the PANORAMA study evaluated aflibercept for the treatment of eyes with moderate to severe NPDR. At 52 weeks, 80 percent of patients achieved two-step regression in DRSS with eight-week dosing of aflibercept. Perhaps more importantly for clinical practice was the reduction in vision threatening complications. Compared with sham, the aflibercept arms had a 75 percent relative risk reduction in the development of proliferative disease or center-involved DME.17
Bottom line
The totality of these data suggest that early intervention in the treatment of diabetic retinal disease is important to provide the best outcomes. Furthermore, consistent and frequent treatments are important, especially in eyes with DME, to sustain the benefit. We acknowledge that there are challenges in maintaining treatment schedules, and current research on long-term delivery applications hopefully will address this unmet need to provide the best treatment for our patients.
REFERENCES
1. Yau JW, Rogers SL, Kawasaki R, Lamoureux EL, Kowalski JW, Bek T, et al. Global prevalence and major risk factors of diabetic retinopathy. Diabetes Care. 2012;35:556–564.
2. Brown DM, Nguyen QD, Marcus DM, et al; RIDE and RISE Research Group. Long-term outcomes of ranibizumab therapy for diabetic macular edema: the 36-month results from two phase III trials: RISE and RIDE. Ophthalmology. 2013 ;120:2013-2022.
3. Wykoff CC, Marcus DM, Midena E, et al. Intravitreal aflibercept injection in eyes with substantial vision loss after laser photocoagulation for diabetic macular edema: Subanalysis of the VISTA and VIVID randomized clinical trials. JAMA Ophthalmol. 2017;135:107-114.
4. Sophie R, Lu N, Compochiaro PA. Baseline predictors of functional outcomes in patients with diabetic macular edema (DME) in the RISE and RIDE trials. Invest Ophthalmol Vis Sci. 2014;55:1702.
5. Baker CW, Glassman AR, Beaulieu WT, et al, for the DRCR Retina Network. Effect of initial management with aflibercept vs laser photocoagulation vs observation on vision loss among patients with diabetic macular edema involving the center of the macula and good visual acuity: A randomized clinical trial. JAMA. 2019 Apr 29. [Epub ahead of print]
6. Ciulla TA, Bracha P, Pollack J, Williams DF. Real-world outcomes of anti-vascular endothelial growth factor therapy in diabetic macular edema in the United States. Ophthalmol Retina. 2018;2:1179-1187.
7. Campbell J, Cole AL, Almony A, et al. Real world vision outcomes in DME treated with anti-VEGF injections—an analysis of EMR data from a large health system. Invest Ophthalmol Vis Sci. 2014;55:3065.
8. Moshfeghi AA, Pitcher JD, Lucas G, Boucher GS, Saroj N. Outcomes of anti-VEGF therapy for neovascular age-related macular degeneration in routine clinical practice. Presented at American Society of Retina Specialists annual meeting; Vancouver, BC; July 21, 2018.
9. Ciulla TA, Hussain RM, Pollack JS, Williams DF. Visual acuity outcomes and anti-vascular endothelial growth factor therapy intensity in neovascular AMD patients: A “real world” analysis in 49,485 eyes. Ophthalmol Retina. Article in press.
10. Early Treatment Diabetic Retinopathy Study Research Group. Grading diabetic retinopathy from stereoscopic color fundus photographs: an extension of the modified Airlie House classification. ETDRS report 10. Ophthalmology. 1991;98:786–806.
11. Diabetic Retinopathy PPP – Updated 2017. American Academy of Ophthalmology website. Updated December 2017. https://www.aao.org/preferred-practice-pattern/diabetic-retinopathy-ppp-updated-2017
12. Deschler EK, Sun JK, Silva PS. Side-effects and complications of laser treatment in diabetic retinal disease. Sem Ophthalmol. 2014;29:290-300
13. Mistry H, Auguste P, Lois N, Waugh N. Diabetic retinopathy and the use of laser photocoagulation: is it cost-effective to treat early? BMJ Open Ophthalmol. 2017 Sep 25;2:e000021.
14. Writing Committee for the Diabetic Retinopathy Clinical Research Network. Gross JG, Glassman AR, Jampol LM, et al. Panretinal photocoagulation vs intravitreous ranibizumab for proliferative diabetic retinopathy: a randomized clinical trial. JAMA. 2015;314:2137–2146.
15. Sivaprasad S, Hykin P, Prevost AT, et al. Intravitreal aflibercept compared with panretinal photocoagulation for proliferative diabetic retinopathy: the CLARITY non-inferiority RCT. Southampton (UK): NIHR Journals Library; 2018 Oct.
16. Wykoff CC, Eichenbaum DA, Roth DB, Hill L, Fung AE, Haskova Z. Ranibizumab induces regression of diabetic retinopathy in most patients at high risk of progression to proliferative diabetic retinopathy. Ophthalmol Retina. 2018;2:997-1009.
17. Brown DM. Intravitreal aflibercept injection (IAI) for moderately severe to severe nonproliferative diabetic retinopathy (NPDR): The Phase 3 PANORAMA Study. Invest Ophthalmol Vis Sci. 2018;59:1889.



