 |
| Dr. Rosenfeld is professor at Bascom Palmer Eye Institute, University of Miami Miller School of Medicine. He has been the principal investigator and study chair for several clinical trials involving wet and dry AMD. Dr. Ramenaden is a medical retina fellow at Bascom Palmer. |
When we analyzed the subjects in these two studies who lost or gained at least three lines of vision after two years, we found that the color fundus, fluorescein angiographic (FA) and optical coherence tomography (OCT) images from eyes with vision loss showed dry lesions without fluorescein leakage characterized as atrophic scars. At that time, we proposed that vision loss after anti-VEGF was unlike vision loss associated with previous therapies used to treat wet AMD.
Before ranibizumab, vision loss resulted from fibrotic scars; but now with ranibizumab, vision loss results from atrophic scars.7 Possible explanations included a subset of patients in whom normal disease progression ensued once the macula was dried or who were exquisitely sensitive to anti-VEGF therapy and progressed to macular atrophy because of the therapy. Interestingly, in all subsequent anti-VEGF trials using monthly dosing, as in the ANCHOR and MARINA trials, about 10 percent of patients lost at least three lines of vision after two years.
Geographic Atrophy and the CATT Trial
In the CATT trial, which compared intravitreal bevacizumab (Avastin, Genentech) with ranibizumab, both anti-VEGF drugs were beneficial, but eyes that had monthly injections exhibited more geographic atrophy (GA) than as-needed (PRN) injections regardless of the drugs. Moreover, ranibizumab was associated with a significantly higher risk of forming GA than bevacizumab regardless of treatment regimen.4Juan E. Grunwald, MD, and colleagues evaluated risk factors for development of GA using color fundus, FA and OCT images from trial patients. They found that a higher rate of residual fluid on OCT corresponded to a lower the rate of GA, which suggested that excessive drying of the macula might promote development of GA.8 Additional evidence from CATT also supported the effect of dosing frequency
on GA formation.
When subjects were switched from monthly to PRN injections after year one, the switched group had lower rates of GA under PRN therapy. Moreover, this lower rate was similar to the second-year rate of GA in those who received PRN therapy for two years but lower than the second-year rate of GA in subjects who continued with monthly therapy in the second year.8
Notably, visual acuities in year two were similar in the ranibizumab and bevacizumab groups, presumably because most of the GA that developed did not involve the central macula. Multivariate analysis identified baseline risk factors associated with GA development to be visual acuity <20/200, retinal angiomatous proliferation (RAP) lesions, GA in the fellow eye and intraretinal fluid.8
A Closer Look at GA
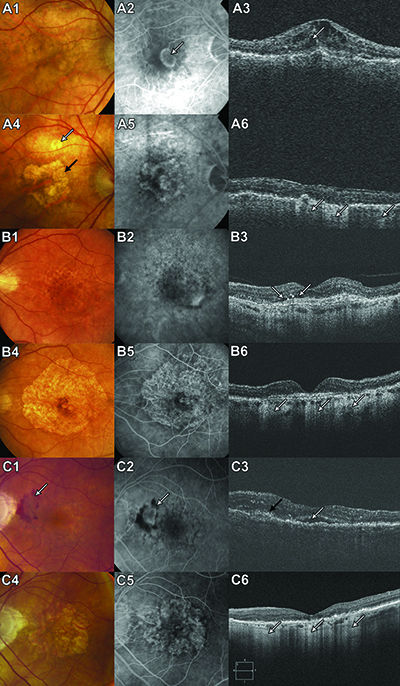 |
GA growth rates were higher with each millimeter the GA margin was from the foveal center, or with greater proximity of GA to the MNV, a history of GA in the fellow eye and the presence of predominantly classic lesions rather than minimally classic and occult lesions.9 A retrospective study of GA in patients who received bevacizumab, ranibizumab and/or aflibercept (Eylea, Regeneron) by Luna Xu, MD, and colleagues, also found that the risk for GA was significantly higher in RAP lesions (type 3 MNV) and lower in occult lesions. They also reported a trend toward GA with greater number of injections, but it was not statistically significant.10
Gui-Shuang Ying, PhD, and colleagues performed another retrospective cohort analysis of the CATT data looking at patient factors that led to sustained vision loss at two years. Bevacizumab treatment was among the baseline factors independently associated with a significantly higher incidence of sustained vision loss. However, they found foveal scarring tended to cause vision loss more often in the bevacizumab-treated eyes while foveal GA tended to be the cause more often in the ranibizumab-treated eyes. However, these findings were not statistically significant. This study did not find any statistical difference between the monthly and PRN dosing groups.11
IVAN was another large prospective clinical trial comparing continuous and discontinuous regimens with bevacizumab and ranibizumab. Unlike the CATT cohort analysis, the percentage of participants with incident GA in IVAN did not differ significantly between drug groups. However, the IVAN analysis did show significantly higher rates of GA with continuous treatment compared with discontinuous treatment, which was similar to the CATT findings.5
A small retrospective study by Erika Tanaka, MD, and colleagues followed eyes with AMD and MNV for 3.5 years, longer than CATT or IVAN. They reported that GA did not develop outside the original boundaries of MNV during anti-VEGF treatment unless the eye had GA outside these boundaries at baseline. They also showed that eyes demonstrating enlargement of GA outside the original MNV boundaries appeared to manifest enlargement of preexisting GA, just as fellow eyes did when they observed the natural evolution of GA in the absence of MNV.12
Similarly, in a retrospective case series analyzing FA and OCT images, Roomasa Channa, MD, and colleagues reported that a majority of eyes developing GA after anti-VEGF therapy did so in areas occupied by MNV while in other eyes GA was present before treatment started. They found that no eyes developed atrophy outside of or adjacent to areas of prior MNV.13 Whether autofluorescence would be better imaging for studying GA progression in eyes undergoing anti-VEGF, as Nishant Kumar, MBBS, and colleagues suggested, remains to be seen.14
Why Does GA Form?
Despite existing prospective and retrospective studies, it remains unclear whether the GA that develops in patients undergoing anti-VEGF therapy secondary to neovascular AMD results from macular drying followed by normal disease progression, or whether VEGF inhibition has a neurotoxic effect on the macula, thus causing the more frequent appearance and more rapid growth of GA.We await the formal publication of the HARBOR Study, in which 2 mg ranibizumab and 0.5 mg ranibizumab were compared using both monthly and PRN regimens. While no data is available on aflibercept and the incidence of GA in the VIEW trials, an ongoing study in Australia comparing ranibizumab and aflibercept to determine whether one causes more GA than the other in neovascular AMD should prove helpful in resolving this question.
As we debate whether anti-VEGF therapy causes GA, there is no question about the efficacy of anti-VEGF therapy for the treatment of MNV in AMD, and we should not withhold treatment. The goal continues to be a dry macula. However, once the macula is dry and GA formation is confirmed, the challenge we have is whether we should consider a less aggressive regimen. This question of anti-VEGF therapy and GA will have particular relevance in the future when sustained-release formulations reach our clinics. RS
Disclosure: Dr. Rosenfeld disclosed he is a consultant to Genentech.
References
1. Brown DM, Kaiser PK, Michels M, Soubrane G, et al. Ranibizumab versus verteporfin for neovascular age-related macular degeneration. N Engl J Med. 2006, 355:1432-1444.2. Rosenfeld PJ, Brown DM, Heier JS, et al. Ranibizumab for neovascular age-related macular degeneration. N Engl J Med. 2006;355:1419-1431.
3. Heier JS, Brown DM, Chong V, et al. Intravitreal aflibercept (VEGF trap-eye) in wet age-related macular degeneration. Ophthalmology. 2012;119:2537-2548.
4. Martin DF, Maguire MG, Fine SL, et al. Ranibizumab and bevacizumab for treatment of neovascular age-related macular degeneration: two-year results. Ophthalmology. 2012;119:1388-1398.
5. Chakravarthy U, Harding SP, Rogers CA, et al. Alternative treatments to inhibit VEGF in age-related choroidal neovascularisation: 2-year findings of the IVAN randomised controlled trial. Lancet. 2013;382:1258-1267.
6. Brown DM, Michels M, Kaiser PK, et al. Ranibizumab versus verteporfin photodynamic therapy for neovascular age-related macular degeneration: Two-year results of the ANCHOR study. Ophthalmology. 2009;116:57-65 e55.
7. Rosenfeld PJ, Shapiro H, Tuomi L, et al. Characteristics of patients losing vision after 2 years of monthly dosing in the phase III ranibizumab clinical trials. Ophthalmology. 2011;118:523-530.
8. Grunwald JE, Daniel E, Huang J, et al. Risk of geographic atrophy in the Comparison of Age-related Macular Degeneration Treatments Trials. Ophthalmology. 2014;121:150-161.
9. Grunwald JE, Pistilli M, Ying GS, et al. Growth of geographic atrophy in the Comparison of Age-related Macular Degeneration Treatments Trials. Ophthalmology. 2014. Dec. 24. [E pub ahead of print]
10. Xu L, Mrejen S, Jung JJ, et al. Geographic atrophy in patients receiving anti-vascular endothelial growth factor for neovascular age-related macular degeneration. Retina. 2015;35:176-186.
11. Ying GS, Kim BJ, Maguire MG, et al. Sustained visual acuity loss in the comparison of age-related macular degeneration treatments trials. JAMA Ophthalmology. 2014;132:915-921.
12. Tanaka E, Chaikitmongkol V, Bressler SB, Bressler NM. Vision-threatening lesions developing with longer-term follow-up after treatment of neovascular age-related macular degeneration. Ophthalmology. 2015;122:153-161.
13. Channa R, Sophie R, Bagheri S, et al. Regression of choroidal neovascularization results in macular atrophy in anti-vascular endothelial growth factor-treated eyes. Am J Ophthalmol. 2015;159:9-19.
14. Kumar N, Mrejen S, Fung AT, et al. Retinal pigment epithelial cell loss assessed by fundus autofluorescence imaging in neovascular age-related macular degeneration. Ophthalmology. 2013;120:334-341.



