 |
|
Bios Dr. Felfeli is an ophthalmology resident at the University of Toronto. Dr. Mandelcorn is an associate professor of ophthalmology at the University of Toronto. DISCLOSURES: The authors have no relevant financial disclosures. |
With the recent news of Google investing $300 million to compete against the artificial intelligence chatbot ChatGPT, we decided to put together a list of some of the exciting advances of artificial intelligence in vitreoretinal surgery. Although it would certainly be convenient to use ChatGPT to generate this article (box below), we conducted our own research on this topic. Here’s what we found in five different pathology areas and about how AI is impacting surgical training and assistance.
Diabetic retinopathy
Diabetic retinopathy screening has shown great potential to benefit from the integration of AI. Devices for AI-based DR grading can function in two modes:1
• Assistive (augmented intelligence) mode. In this mode, an ophthalmologist reviews the grading the device provides and can adjust or accept it based on their level of agreement.
• Fully automatic mode. This mode doesn’t allow for ophthalmologist review of the images. Based on the evaluation of multimodal images, the machine sends referable DR and ungradeable images directly to the ophthalmologist.
Studies have shown promising results for the use of these technologies, particularly in countries with high diabetes rates.2 In countries such as Singapore, analyses have estimated that this technology will produce yearly cost savings upwards of $15 million by 2050.3
This technology won’t only benefit vitreoretinal surgeons and their patients, but also allow them to screen more patients in a more time-efficient manner, helping them to triage patients who require prompt intervention.
 |
|
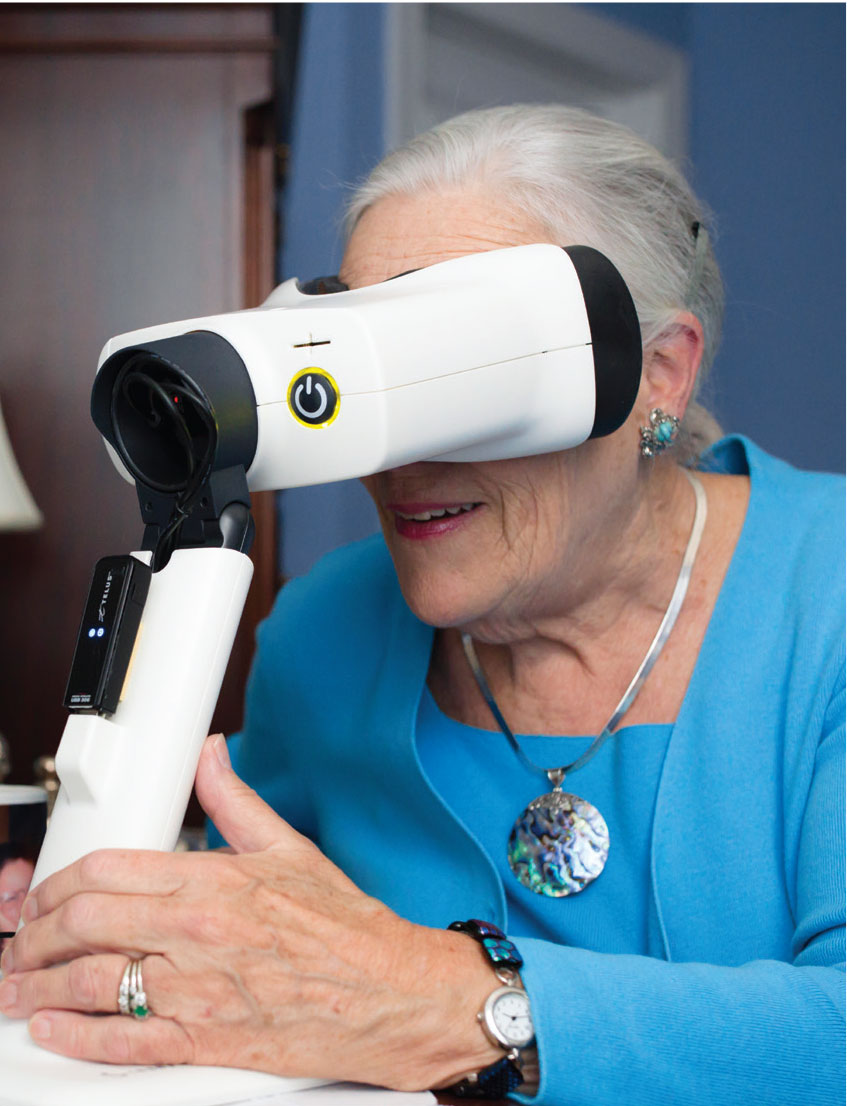
Figure 1. Notal Vision’s ForeseeHome, invented and made in Israel, allows critical at-home daily monitoring of a disease that can quickly lead to vision loss. (Courtesy Notal Vision) |
Age-related macular degeneration
AI algorithms have proven to be effective in detecting age-related macular degeneration using optical coherence tomography images, with a patient-level accuracy of 93 percent.4 The ongoing RAZORBILL study is exploring how AI algorithms can assist in treatment decisions for patients with neovascular AMD by analyzing three-dimensional OCT scans and extracting relevant features for provider review.5
AI can also predict the need for anti-VEGF treatment and a patient’s likely response to therapy.6,7 Based on OCT images, visual acuity and demographic characteristics, it may also play a pivotal role in the discovery of novel treatments for AMD, along with other ophthalmic diseases.
AI has demonstrated utility in the development and design of pharmaceuticals, with applications in molecular design, toxicity prediction and many other areas of the drug design process.8 Additionally, it has been utilized for the complex analysis of intraocular biomarkers and the correlation of cytokines profiles in ocular diseases.9,10
In terms of devices, the ForeseeHome (Notal Vision) AMD monitoring system is a home-based device that patients can operate independently (Figure 1). It uses AI to detect statistically significant changes from a patient’s baseline and alerts providers of these results.11
 |
This device proved to be beneficial in minimizing a decline in visual acuity from baseline to choroidal neovascularization detection.12 Similar to the DR screening applications, this technology allows for optimal management of patients and ensures that patients progressing to a further stage of their disease receive prompt attention and treatment.
Retinal detachment
AI may be applied to ophthalmic ultrasonography to screen patients with retinal detachment.13 AI models can analyze still images and videos of ultrasonography scans and determine if they show an RD, vitreous detachment, vitreous hemorrhage or tears, with an accuracy of 90 percent or higher in all categories.
Deep-learning models also have implications for predicting anatomical outcomes of RD postoperatively with an area under the curve (AUC) of 0.94, sensitivity of 73 percent and a specificity of 96 percent.14
Lastly, a screening algorithm has been developed to analyze ultra-widefield fundus images for RD detection, including the distinction between macula-on and macula-off RD.15 Interestingly, the algorithm could also provide guidance to patients regarding head positioning, which could minimize the progression of their RD prior to surgical repair.
ChatGPT’s AI-generated response about AI’s potential in vitreoretinal surgery ChatGPT prompt: Regarding artificial intelligence for vitreoretinal surgeons, what is in the pipeline and how will it change their practices? AI-generated response: There is a growing interest in the use of artificial intelligence (AI) in vitreoretinal surgery, as it has the potential to improve surgical outcomes and increase efficiency. The following are some examples of AI applications in this field: 1. Image analysis: AI algorithms can be trained to analyze retinal images to identify features such as blood vessels, retinal detachments and age-related macular degeneration. This information can assist surgeons in making more informed decisions about the best treatment approach. 2. Surgical planning and navigation: AI can be used to assist in surgical planning by analyzing images of the eye and creating a personalized surgical plan based on the anatomy of the eye and the specific disease being treated. Additionally, AI-powered navigation systems can be used during surgery to help guide the surgeon and improve accuracy. 3. Predictive modeling: AI algorithms can be trained to predict the outcomes of surgical procedures, allowing surgeons to make informed decisions about the best approach for each patient. 4. Real-time surgical assistance: AI can be integrated into surgical equipment, such as handheld devices, to provide real-time feedback and guidance during surgery. This can improve accuracy and safety, and reduce the time needed for each procedure. Overall, AI has the potential to greatly enhance the practice of vitreoretinal surgery by improving surgical outcomes, increasing efficiency, and reducing the risk of complications. As more AI applications are developed and validated, it is likely that they will become an increasingly important tool for vitreoretinal surgeons. |
Macular hole
Studies of AI applications in macular hole diagnosis and management show great promise and represent a crucial step toward the widespread adoption of this technology in the future. Researchers in China developed a deep-learning model to predict postoperative macular hole status after internal limiting membrane peeling.16
The model made these predictions using preoperative OCT images and achieved an accuracy of 84.7 percent in external validation, with an AUC of 89.32 percent (sensitivity of 83.33 percent and specificity of 87.5 percent). Researchers in Italy explored the potential of AI to predict the one-year visual acuity of eyes affected by full-thickness macular hole.17 The predictions were based on preoperative OCT-A scans and the researchers evaluated various AI models to improve the results.
These studies highlight the potential of AI in predicting postoperative outcomes, which could help guide decision-making between providers and patients.
 |
|
Figure 2. Artificial intelligence-generated art provided courtesy of DALL-E 2, using the prompt, “3D rendering of vitreoretinal surgeon using artificial intelligence while doing eye surgery.” |
Epiretinal membrane
 |
The applications of AI for diagnosis and management of epiretinal membrane have also been demonstrated. A recent study aimed to validate the use of AI to diagnose an ERM based on color fundus photographs.18 The AI model produced encouraging results, with comparable accuracy and a higher sensitivity than manual diagnosis by an ophthalmologist based on fundus photographs alone (77.08 vs. 75.69 percent and 75.9 vs. 63.86 percent, respectively). Notably, the AI model had a lower specificity when compared with manual diagnosis (78.69 vs. 91.8 percent).
AI has also demonstrated its use for predicting long-term visual improvement after ERM surgery, based on OCT images.19 The-deep learning model achieved a sensitivity of 87.3 percent and a specificity of 86.2 percent when predicting one-year visual outcomes for ERM patients who underwent surgical intervention.
Surgical training and assistance
The field of vitreoretinal surgery has seen significant advancements in AI with the integration of robotics, such as tremor reduction, other surgical assistive functions and surgical training devices (Figure 2).20
One study demonstrated that AI can be used in vitreoretinal procedures to localize, classify and segment instruments and tissues in real time, which may allow for prevention of surgical errors in the future.21 This kind of technology is a step toward making microsurgeries much safer and more precise.
Additionally, researchers at Purdue University described the AI-Medic, an AI mentor for trauma surgery that envisions autonomous medical mentoring by using computer vision and AI algorithms to provide instructions to surgeons when a reliable communication medium with an expert isn’t available.22
Similar computer vision technology may be used in the future to evaluate a vitreoretinal surgeon’s performance and to provide feedback on their technique.23
AI may also be used to track surgical instruments during operations.24 This could have interesting applications for optimizing workflow in vitreoretinal surgeries. For example, AI may be able to predict which piece of equipment will be required in the next step of a surgery and aid in providing it in a timely manner. AI may also be used to ensure that certain steps aren’t omitted during a procedure.
Bottom line
Many exciting applications of AI for vitreoretinal surgeons are already here and on the horizon. We agree with ChatGPT’s statement: “AI has the potential to greatly enhance the practice of vitreoretinal surgery by improving surgical outcomes, increasing efficiency, and reducing the risk of complications.”
However, a key consideration will be how we implement this technology in an equitable manner to ensure that it’s available for all patients, including disadvantaged populations. RS
REFERENCES
1. Raman R, Dasgupta D, Ramasamy K, George R, Mohan V, Ting D. Using artificial intelligence for diabetic retinopathy screening: Policy implications. Indian J Ophthalmol. 2021;69:2993–2998.
2. Gulshan V, Rajan RP, Widner K, et al. Performance of a deep-learning algorithm vs manual grading for detecting diabetic retinopathy in India. JAMA Ophthalmol. 2019;137:987–993.
3. Xie Y, Nguyen QD, Hamzah H, et al. Artificial intelligence for teleophthalmology-based diabetic retinopathy screening in a national programme: an economic analysis modelling study. Lancet Digit Health. 2020;2:e240–249.
4. Lee CS, Baughman DM, Lee AY. Deep learning is effective for the classification of OCT images of normal versus age-related macular degeneration. Ophthalmol Retina. 2017;1:322–327.
5. Holz FG, Abreu-Gonzalez R, Bandello F, et al. Does real-time artificial intelligence-based visual pathology enhancement of three-dimensional optical coherence tomography scans optimise treatment decision in patients with nAMD? Rationale and design of the RAZORBILL study. Br J Ophthalmol. 2023;107:96–101.
6. Bogunovic H, Waldstein SM, Schlegl T, et al. Prediction of anti-VEGF treatment requirements in neovascular AMD using a machine learning approach. Invest Ophthalmol Vis Sci. 2017;58:3240–3248.
7. Bogunovic H, Abramoff M, Zhang L, Sonka M. Prediction of treatment response from retinal OCT in patients with exudative age-related macular degeneration. Ophthalmic Medical Image Analysis International Workshop 1(2014), 129-136. doi: https://doi.org/10.17077/omia.1018
8. Gupta R, Srivastava D, Sahu M, Tiwari S, Ambasta RK, Kumar P. Artificial intelligence to deep learning: machine intelligence approach for drug discovery. Mol Divers. 2021;25:1315–1360.
9. Nezu N, Usui Y, Saito A, et al. Machine learning approach for intraocular disease prediction based on aqueous humor immune mediator profiles. Ophthalmology. 2021;128:1197–1208.
10. Pucchio A, Krance SH, Pur DR, Miranda RN, Felfeli T. Artificial intelligence analysis of biofluid markers in age-related macular degeneration: A systematic review. Clin Ophthalmol. 2022;16:2463–2476.
11. Ho AC, Heier JS, Holekamp NM, Garfinkel RA, Ladd B, Awh CC, et al. Real-world performance of a self-operated home monitoring system for early detection of neovascular age-related macular degeneration. J Clin Med. 2021;10:1355.
12. Brucker AJ, AREDS II -HOME STUDY. The Home Monitoring of the Eye (HOME) study: Potential implication of findings on management of intermediate AMD patients. Invest Ophthalmol Vis Sci. 2014 ;55:3067.
13. Chen D, Yu Y, Zhou Y, et al. A deep learning model for screening multiple abnormal findings in ophthalmic ultrasonography (with video). Translational Vis Sci Tecj. 2021;10:22.
14. Fung THM, John NCRA, Guillemaut JY, et al. Artificial intelligence using deep learning to predict the anatomical outcome of rhegmatogenous retinal detachment surgery: A pilot study. Graefes Arch Clin Exp Ophthalmol. Published online October 28, 2022. Available at: https://doi.org/10.1007/s00417-022-05884-3
15. Li Z, Guo C, Nie D, et al. Deep learning for detecting retinal detachment and discerning macular status using ultra-widefield fundus images. Commun Biol. 2020;3:1–10.
16. Hu Y, Xiao Y, Quan W, et al. A multi-center study of prediction of macular hole status after vitrectomy and internal limiting membrane peeling by a deep learning model. Ann Transl Med. 2021;9:51.
17. Rizzo S, Savastano A, Lenkowicz J, et al. Artificial intelligence and OCT angiography in full thickness macular hole. New developments for personalized medicine. Diagnostics (Basel). 2021;11:2319.
18. Shao E, Liu C, Wang L, et al. Artificial intelligence-based detection of epimacular membrane from color fundus photographs. Sci Rep. 2021;11:19291.
19. Crincoli E, Savastano MC, Savastano A, et al. New artificial intelligence analysis for prediction of long-term visual improvement after epiretinal membrane surgery. Retina. 2024:43:173-181.
20. Gerber MJ, Hubschman JP. Robotics in vitreoretinal surgeries. In: Jain A, Natarajan S, Saxena S, eds. Cutting-edge Vitreoretinal Surgery. Singapore: Springer Singapore. 2021: 89–99.
21. Nespolo RG, Yi D, Cole E, Wang D, Warren A, Leiderman YI. Feature tracking and segmentation in real time via deep learning in vitreoretinal surgery-a platform for artificial intelligence-mediated surgical guidance. Ophthalmol Retina. Published online October 12, 2022. Available at: https://www.sciencedirect.com/science/article/abs/pii/S2468653022004882?via%3Dihub
22. Rojas-Muñoz E, Couperus K, Wachs JP. The AI-Medic: An artificial intelligent mentor for trauma surgery. Comput Methods Biomech Biomed Eng: Imaging Visualization. 2021;9:313–321.
23. Bakshi SK, Lin SR, Ting DSW, Chiang MF, Chodosh J. The era of artificial intelligence and virtual reality: transforming surgical education in ophthalmology. Br J Ophthalmol. 2021;105:1325–1328.
24. Nema S, Vachhani L. Surgical instrument detection and tracking technologies: Automating dataset labeling for surgical skill assessment. Frontiers Robotics AI. 2022;9:1030846.




