
  |
Voretigene neparvovec-rzyl (Luxturna, Spark Therapeutics) is the first gene therapy approved for ocular use in patients with RPE65-mediated inherited retinal dystrophy. A Phase III trial found that the treatment was well-tolerated and resulted in a significant improvement in multi-luminance mobility testing. The drug, delivered in the subretinal space, has been administered in several centers.
Here, we hold a clinical conversation on the technical aspects of administering voretigene neparvovec-rzyl. On page 24, we also provide the perspective of the mother of one of our patients.
Choosing instrumentation
Nicolas Yannuzzi, MD: What gauge instrumentation do you use for these cases?
Audina Berrocal, MD: I use the 25-gauge EVA vitrectomy pack (DORC) system.
NY: Posterior vitreous detachment (PVD) induction can be challenging in children. Do you typically use a staining agent or other instruments besides the vitreous cutter to lift the hyaloid?
AB: I stain all these cases because I want to be completely sure that I’ve elevated the hyaloid. I generally start by trying to induce a PVD without kenalog, then inject the kenalog later for confirmation. In a 2-year old, the youngest child I have treated so far, the vitreous behaved more like the vitreous of a normal child. It was very adherent at the macula, so I used a flex loop to tease the vitreous away from the macula, then worked the PVD out more peripherally with the cutter.
NY: How aggressive are you in shaving the peripheral vitreous?
AB: I try to elevate the hyaloid as far peripherally as possible, then I trim it with the cutter, but I don’t typically do a 360-degree depressed shave. However, I do depress 360 degrees to look for any abnormalities or retinal breaks as I trim the vitreous. You don’t want to have an unrecognized retinal break or detachment in these children. I would rather treat anything suspicious in the periphery with laser since I’m already in the eye. Thus far I have observed only one small retinal tuft in one eye and I treated it.
NY: How is the vitreoretinal interface in these patients?
AB: The younger the patient, the more normal the posterior adhesion is. Usually in the periphery the vitreous tends to be less adherent. Older children with more significant disease are easier to elevate.
Using the microinjector
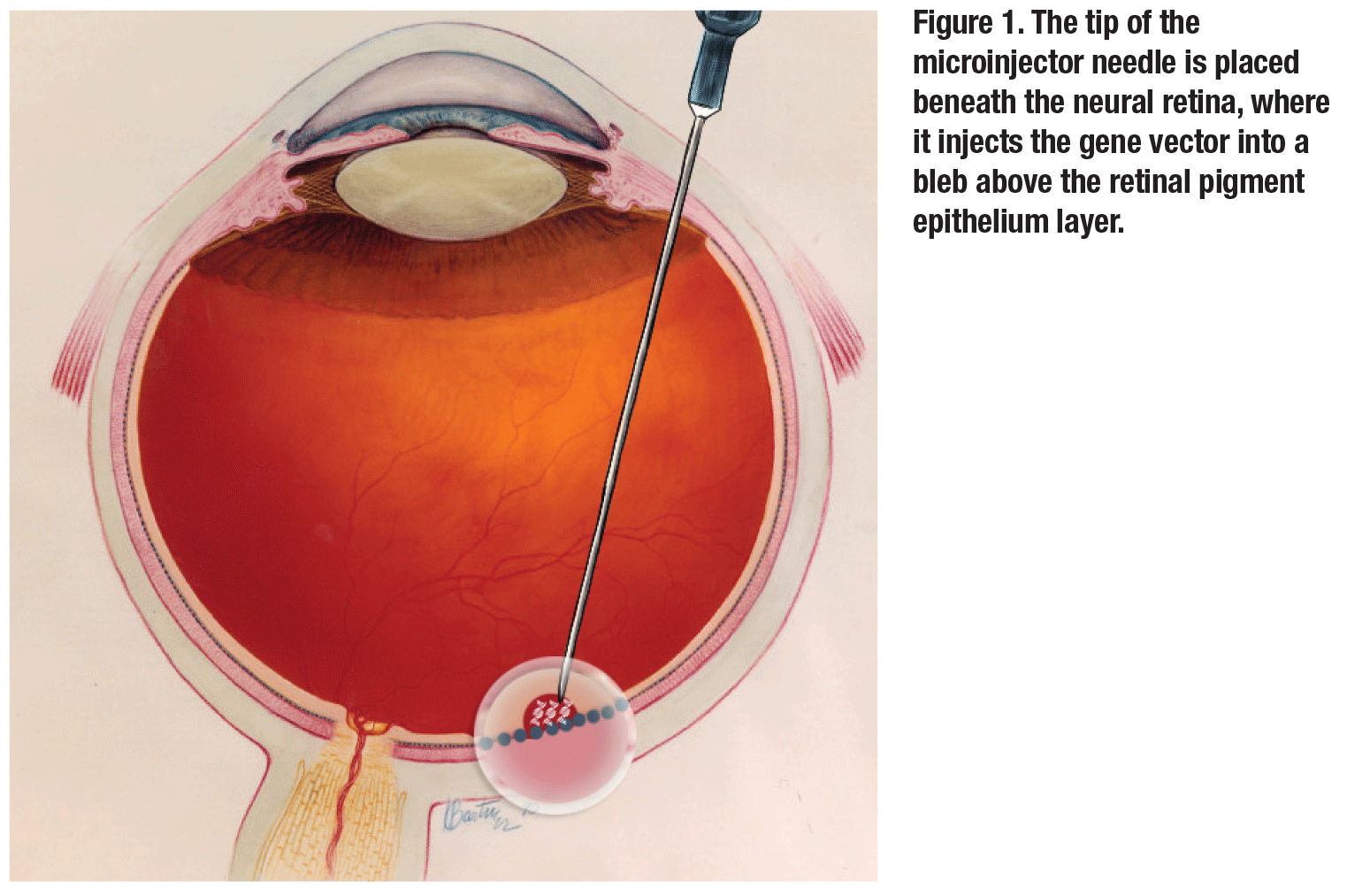 |
NY: You did your first several cases by injecting manually with an assistant. Since then, you’ve transitioned to the MicroDose microinjector (MedOne). Do you find this to be a more controlled way of delivering the drug?
AB: Definitely there’s better control. The injection pressure is known, and you don’t have the added variable of not knowing how hard the assistant is pushing. Too high of an injection pressure can cause trauma to the retina or retinal pigment epithelium. The MedOne micro-injector kit works great and it makes you an independent surgeon, which is always more controlled.
NY: There are differences in opinions on whether to bevel the needle for the injector. What has been your experience?
AB: I’ve tried using the injector non-beveled, but haven’t been that successful in entering the subretinal space. Beveling it allows entry in a more elegant way.
Usefulness of intraoperative OCT
 |
NY: Do you routinely use intraoperative optical coherence tomography for these cases?
AB: For these cases, intraoperative OCT is a must. It allows you to confirm the extent of the bleb (both vertically and horizontally), the location of the medication and whether the fovea has been detached. In the clinical trial, among children that were treated, there was one case of iatrogenic macular hole. By using the OCT, I can also confirm no hole has been induced.
NY: How much volume do you inject and where do you typically aim to make your blebs?
AB: You want to inject 0.3 ml, as was done in the clinical trial. Many times this can’t be done in a single bleb. If the retina becomes too elevated or thin, it’s possible to create a hole. As I perform the injection, I use OCT to get a feel for the height of the bleb. Ideally I start within the arcades to ensure some uptake in the macula. I will occasionally form two to four communicating blebs or non-communicating blebs.
Macular and foveal detachment
NY: Do you think macular and foveal
detachment is necessary for success?
AB: There are different schools of thought about this. The clinical trial required detachment at the macula. But what I’ve noticed is that you can get close to it and even if it doesn’t occur completely, you can still have a good result. Sometimes, I’ll lift two separate blebs that eventually communicate with each other at the macula.
NY: What tamponade do you use for these cases?
AB: The trial used an air-fluid exchange to eliminate the virus from the preretinal space and decrease inflammation. That’s what I’ve been doing in all my cases.
Steroid therapy
NY: What steroids are you using pre-, intra- and postoperatively?
AB: I prescribe 1 mg/kg per day of oral prednisone starting three days before the surgery and continue this until 10 days after the second eye. Ideally, I space each eye apart between seven and 18 days. I also give the child a sub-Tenon’s kenalog injection at the end of surgery and topical steroids postoperatively. I’ve observed some moderate steroid responses, but all of my patients have been controlled on topical glaucoma agents, and their [intraocular] pressure normalized off medications after the steroids were tapered.
 |
Measuring success, recovery
NY: In children, visual acuity and other objective outcome measures can be difficult to ascertain. How can you measure success in these cases?
AB: Success is really seeing these kids need less light and less help and having surgery without complications. The clinical trial never used Snellen acuity as an endpoint. We‘re looking more at function. Doing visual fields in kids is impossible. We hope to do electroretinograms at follow-up visits. Contrast sensitivity is important, but it’s very challenging to test a 2-year-old in the clinic.
NY: What have you noticed about these children during recovery?
AB: Three days after you inject the vector, parents already notice a change in the amount of light their children need. Imagine that three days after you inject the viral vector, retinal cell biology is changing. That to me is magic. It’s almost like science fiction. As the weeks go by, parents send me videos and tell me about the amount of change they notice in the lives of their children.
NY: What do you think about visual development in these children?
AB: Studies have shown there’s a lot of neural plasticity after the viral injection in these children. Patching is a must. I work with pediatric ophthalmologists for visual rehabilitation, and all of my colleagues have been attempting patching. Remember, many of these children haven’t been tested visually for years or have not had amblyopia management because of their underlying disease.
NY: Any other tips for these cases?
AB: Although I use a noncontact system, I like a contact lens for the subretinal injection to enhance
stereopsis. I suture the sclerotomies at the end of every case and give 20 mg of a sub-Tenon’s kenalog. RS
 |



