 |
 |
A 56-year-old male physician was referred urgently to the University of Washington retina clinic with a one-week history of a “small blind spot” in the right eye. The scotoma was just superior to fixation with a purple and yellow hue.
He recalled a similar scotoma in the left eye 20 years earlier. At that time, an evaluation by an ophthalmologist failed to reveal an etiology.
Otherwise, his ocular history was unremarkable. His medical history was notable for idiopathic myelofibrosis, initially diagnosed in 2001, for which he had undergone a recent splenectomy and matched unrelated donor peripheral blood stem-cell transplantation. His current medications included mycophenolate, cyclosporine and sirolimus.
Examination findings
Best-corrected visual acuity was 20/20 in both eyes. Pupils were equally reactive with no afferent pupillary defect. Intraocular pressures were normal, as were extraocular movements and peripheral visual field by confrontation. On Amsler grid testing, he had a wedge-shaped scotoma superonasal to the point of fixation in the right eye and a fainter wedge-shaped scotoma directly superior to fixation in the left eye. The anterior slit lamp exam was unremarkable.
Fundus examination revealed a subtle interruption of the foveal light reflex in the inferotemporal macula of the right eye, with an otherwise normal macular and peripheral exam (Figure 1).
Work-up
Spectral-domain optical coherence tomography of the right eye revealed a focal area of hyperreflectivity involving the outer plexiform and nuclear layers along with focal irregularity of the ellipsoid zone temporal and inferotemporal to the fovea (Figure 2A). SD-OCT of the left macula showed subtle focal outer retinal thinning and ellipsoid granularity nasal to the fovea (Figure 2A).
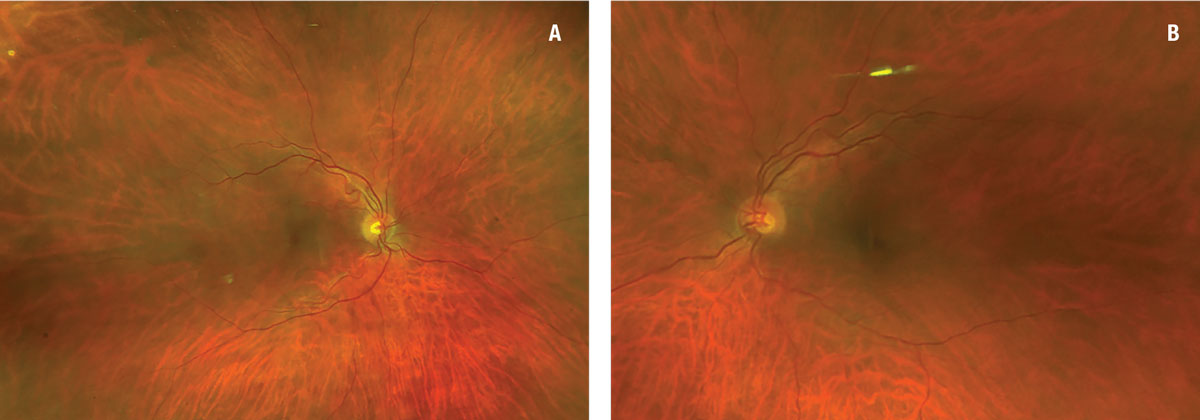 |
| Figure 1. Fundus photography was relatively unremarkable in both eyes, showing a subtle interruption of the foveal light reflex in the inferotemporal macula of the right eye (A) with an otherwise normal macular and peripheral exam (B). |
Near-infrared reflectance demonstrated a lesion with focal hyporeflectance inferotemporal to the fovea of the right eye and inferonasal to the fovea in the left, corresponding to the SD-OCT irregularities (Figure 3). Fluorescein angiogram showed normal perfusion in both eyes (Figure 4).
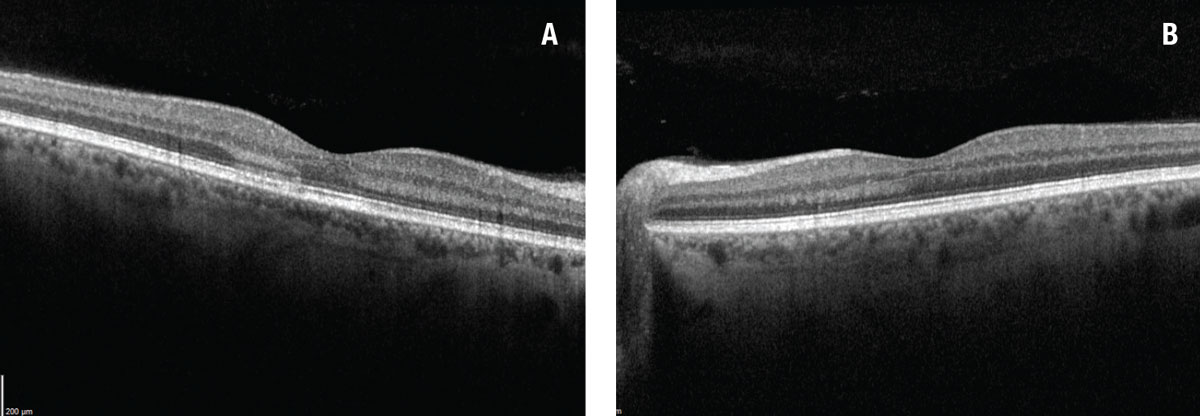 |
| Figure 2. A) Spectral-domain optical coherence tomography of the right eye revealed a focal area of hyperreflectivity within the outer nuclear and plexiform layers along with disruption of the ellipsoid zone. B) SD-OCT in the left eye revealed subtle focal outer retinal thinning and ellipsoid zone granularity nasal to fovea. |
Diagnosis and management
Multimodal imaging demonstrated features consistent with a diagnosis of acute macular neuroretinopathy (AMN). At the one-month follow-up visit, the patient’s symptoms were unchanged. The near infrared reflectance in the right eye showed a more consolidated hyporeflective lesion. SD-OCT showed a slight interval improvement in the ellipsoid zone disruption.
What’s AMN?
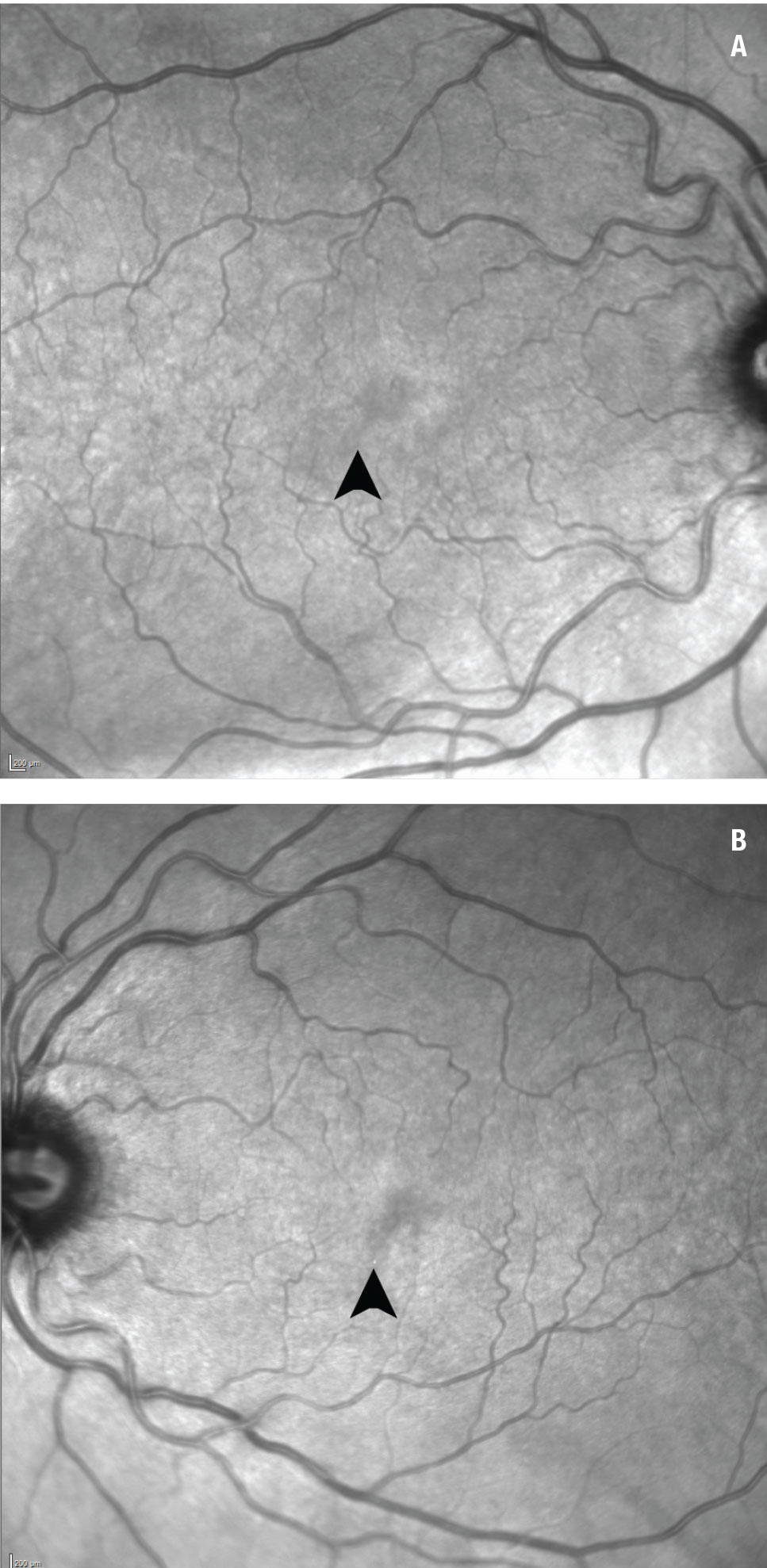 |
| Figure 3. Near-infrared reflectance shows a focal area of hypo-reflectance in the inferotemporal fovea of the right eye (A) and the inferonasal fovea of the left eye (B) (arrows). |
Acute macular neuroretinopathy was first described 45 years ago by Pierre J.M. Bos, MD, and August F. Deutman, MD.1 A subsequent large review of AMN found the typical demographic to be young women in their late 20s, with about half of cases having bilateral ocular involvement.2
The most common presenting symptom was a scotoma, often near the point of fixation, corresponding to wedge-shaped lesions on examination. They have been described to have either a petaloid, oval, or teardrop shape with the apex directed toward the fovea. Occasional associated findings include superficial retinal hemorrhages, macular edema and optic disc edema.
The differential diagnosis for AMN includes white-dot syndromes such as multiple evanescent white-dot syndrome (MEWDS) and acute posterior multifocal placoid pigment epitheliopathy (APMPPE), as well as punctate inner choroidopathy (PIC) and paracentral acute middle maculopathy (PAMM).
Multimodal imaging is useful for establishing the diagnosis in these cases. Infrared reflectivity classically shows perifoveal, eccentric hyporeflective lesions and has been reported to be the most sensitive and specific diagnostic study.2 SD-OCT shows both ellipsoid and/or interdigitation zone disruption with focal overlying outer nerve and plexiform hyperreflectivity.
Fluorescein angiography has low sensitivity, but may show hypofluorescent lesions in either the early or late phases. Multifocal electroretinogram demonstrates diminished amplitudes at the areas corresponding to the patient’s scotoma.
 |
No proven medical or surgical treatment exists for AMN. Scotomas may improve spontaneously over time, but generally don’t resolve completely.
Insights into pathophysiology
The pathophysiology of AMN is undetermined. However, since the advent of OCT angiography, a growing body of evidence suggests that AMN may involve an abnormality of the microvasculature in the choriocapillaris or deep capillary plexus.3
The most common systemic association is a nonspecific preceding febrile illness. However, factors that may cause a disruption to blood flow either through vasoconstriction, decreased cardiac output or a prothrombotic state have been associated with AMN.
Very few associations between hematologic malignancies and AMN have been described. The first noted association between leukemia and AMN was reported in a 31-year-old woman with acute lymphoblastic B-cell leukemia, who presented with six weeks of vision loss.4 A more recent case report described AMN in a patient with newly diagnosed acute promyelocytic leukemia.5
Suggested mechanisms for AMN in these patients included thrombocytopenia and anemia leading to focal ischemia of the outer retina, immature blast cells increasing the overall hyperviscosity of serum leading to venous stasis, and disseminated intravascular coagulation causing worsening of perfusion of the retinal microvasculature.5
Chemotherapy itself has also been linked to AMN. We found one report of AMN in a 28-year-old woman with refractory acute myeloid leukemia (AML) on gilteritinib therapy who was
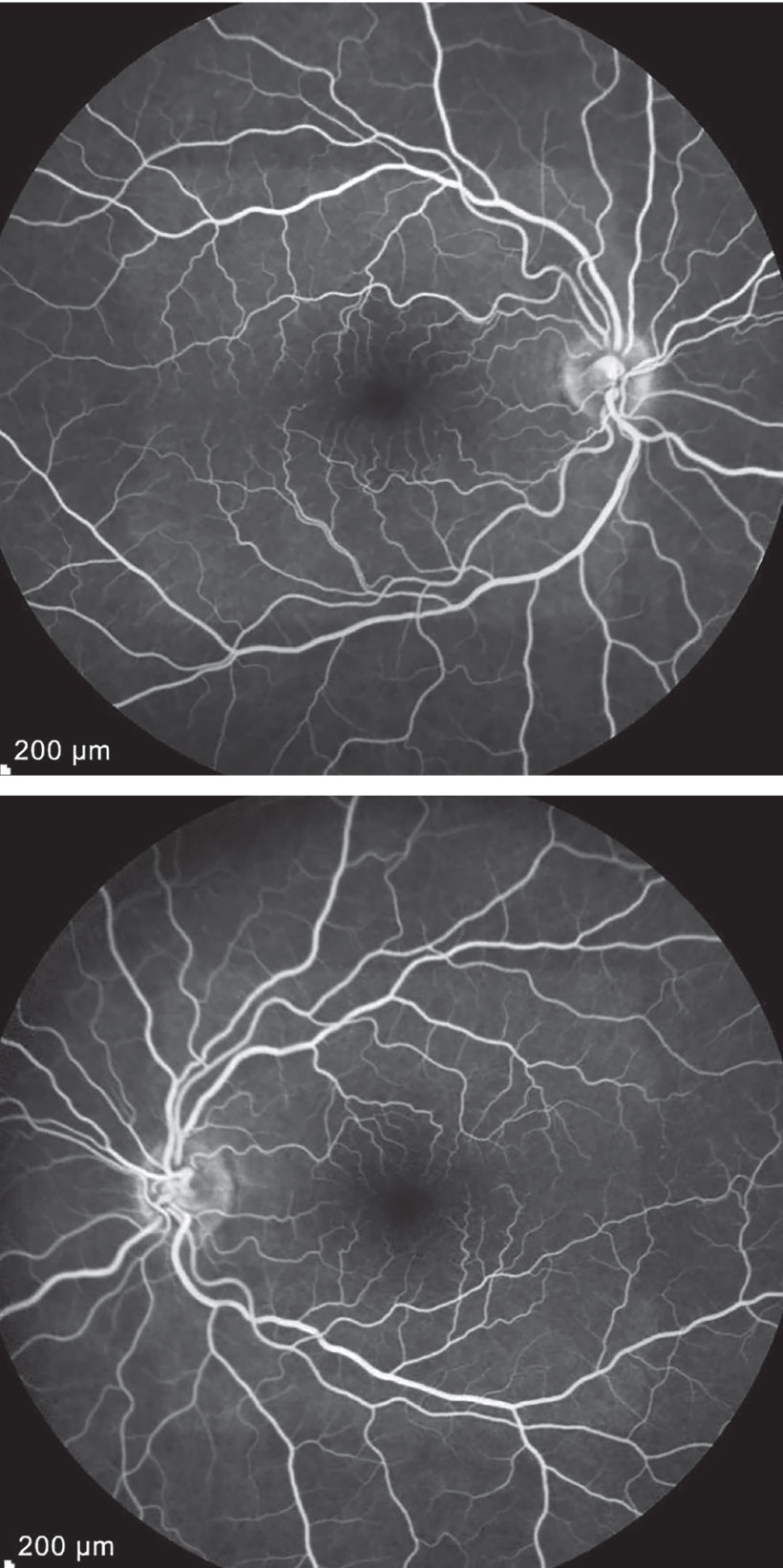 |
| Figure 4. Fluorescein angiography was unremarkable in both eyes. |
diagnosed with AMN.6 She subsequently had an improvement in symptoms after discontinuing the chemotherapy agent. Gilteritinib is an inhibitor of FLT3, and retinal FLT3 is known to be upregulated in ischemic conditions. Mice lacking the FLT3 gene in laboratory conditions have markedly reduced angiogenesis. Gilteritinib may play a role in impairing the retina’s normal response to transient ischemic states and lead to the development of AMN.
Bottom line
To our knowledge, this is the first case of AMN associated with myelofibrosis. Microvascular ischemia at the level of the deep capillary plexus or choriocapillaris can have a wide variety of etiologies, and this report contributes to the case reports that show an association between AMN and a hematologic malignancy.
Interestingly, this patient’s diagnosis of myelofibrosis was established shortly after his initial episode of what was most likely AMN in the contralateral eye. The paracentral scotoma might have been the presenting symptom of his underlying blood dyscrasia.
Because of this, we recommend that clinicians carry an index of suspicion for serious systemic conditions in patients who present with AMN. Consider ordering basic blood work, including a complete blood count with differential, when encountering a patient with potential symptoms of AMN. RS
REFERENCES
1. Bos PJ, Deutman AF. Acute macular neuroretinopathy. Am J Ophthalmol. 1975;80:573-584.
2. Bhavsar KV, Lin S, Rahimy E, et al. Acute macular neuroretinopathy: A comprehensive review of the literature. Survey Ophthalmol. 2016;61:538-565.
3. Thanos A, Faia LJ, Yonekawa Y, Randhawa S. Optical coherence tomographic angiography in acute macular neuroretinopathy. JAMA Ophthalmol. 2016;134:1310-1314.
4. Munk MR, Jampol LM, Souza EC, et al. New associations of classic acute macular neuroretinopathy. Br J Ophthalmol. 2016;100:389-394.
5. Thakar SD, Hassan OM, Gill MK. Acute macular neuroretinopathy associated with acute promyelocytic leukemia. Am J Ophthalmol Case Rep. 2021;22:101044.
6. Liu Y, Haq Z, Pasricha ND, Bever GJ. Acute macular neuroretinopathy associated with an oral FLT3 inhibitor. JAMA Ophthalmol. 2020;138:1104-1106.



