Uveitis is the cause of 10 to 15 percent of the overall cases of blindness in the United States,1 and accounts for 30,000 new cases
 |
of legal blindness each year.2 The incidence of uveitis has been calculated at 25 to 341 cases per 100,000 person years.3-6 Among pediatric patients, males are affected more often than females. However, adult females are affected more than males.
The incidence of uveitis increases with age. Among patients younger than age 18 in the United States, the incidence is approximately 31 cases per 100,000 person years. That quadruples to 133 per 100,000 person years for patients age 18 to 64, and
 |
then almost doubles to 220 per 100,000 patient years for those age 65 and older.7 Outside of tertiary care centers, anterior uveitis accounts for 92 percent of all cases.8 Up to 50 percent of uveitis cases can be associated with an underlying systemic disease.9
In this clinical review, we provide a primer for retina specialists, because they may soon be playing a larger role in the management of uveitis as new drug treatments move toward approval.
Classification and Etiology
Multiple factors define uveitis. They are:10
-location;
-timing of onset;
-duration;
-course;
-laterality; and
-presence of granulomatous characteristics.
• Location. Anterior uveitis, consisting of iritis and iridocyclitis, is the most common form, accounting for 25 to 92
 |
percent of cases.11,12 Posterior uveitis, consisting of choroiditis, retinitis and chorioretinitis is the second most common, accounting for 5 to 38 percent of cases.13 Intermediate uveitis is the third most common, accounting for 1 to 12 percent of cases.2,11 Panuveitis is the least common, accounting for 1 to 9 percent of cases.14
• Characteristics. Uveitis etiology is divided accordingly:
-idiopathic;
-infectious;
-systemic inflammatory;
-hypersensitivity reactions; and
-uveitis syndromes restricted to the eye.
More than 90 percent of cases are noninfectious in nature.7 About 9 percent of adult noninfectious causes are attributable to systemic immune conditions.8
In the United States, most infectious causes of uveitis are decreasing,14 although in many developed counties syphilis has been increasing in prevalence in recent years.15 In anterior uveitis, the most common causes are idiopathic, seronegative spondyloarthropathies, juvenile idiopathic arthritis (JIA) and herpetic disease.16 The most common cause of posterior uveitis is toxoplasmosis.17 Panuveitis is typically idiopathic or due to sarcoidosis, whereas intermediate uveitis is typically idiopathic.12 A comprehensive list of etiologies appears in Table 1.
Workup Step One: History
A detailed history is crucial during the workup of uveitis. Key components are evaluation of symptoms, investigation of medical, social and family histories, and a review of systems.
• Evaluation of symptoms. Symptoms alone can often determine anatomic classification. Symptoms of acute anterior uveitis typically include pain, redness, photophobia and blurry vision. In a chronic case, anterior uveitis may present with minimal pain and photophobia. Intermediate and posterior uveitis typically present with floaters or blurry vision.
Inquire about symptom course, laterality, previous episodes, response to treatment and any associated systemic symptoms.
• Medical and surgical history. Carefully
 |
review prior episodes of trauma, as well as medical and surgical history. Medical history should focus on systemic diseases such as sarcoidosis, JIA, HIV/AIDS, tuberculosis and syphilis. Medication use may be important.
• Social history. This investigation should focus on race/ethnicity, birthplace, travel history, diet, sexual history and history of intravenous drug use.
• Family history. This should involve family history of systemic or ocular diseases, contagious diseases and prenatal infections.
• Review of systems. This final step may reveal symptoms that the patient neglected to report. Table 2 lists items to inquire about.
Conducting the Examination
A careful ophthalmic examination will help to localize the inflammation and may provide clues to the etiology of the disease. Intraocular pressure is usually reduced in acute anterior uveitis due to ciliary body shutdown. Ciliary body atrophy or detachment can occur in chronic cases, which can produce chronic hypotony and eventually phthisis.
Elevated IOP may also result from trabeculitis, inflammatory plugging of the trabecular meshwork, pupillary block, peripheral anterior synechiae or a steroid response. Elevated IOP is particularly associated with herpetic anterior uveitis.16
The examination should focus on the following anatomical regions:
• Conjunctiva. Inspect this structure for nodules (especially in sarcoidosis) or ciliary flush (perilimbal injection), which may appear in anterior uveitis. Conjunctival nodules can be biopsied in cases where the diagnosis is uncertain.
• Cornea. The cornea may also display epithelial dendrites, ulcers or stromal inflammation in herpetic disease. Keratic precipitates (KPs)—collections of inflammatory cells—may be present on the corneal endothelial surface. They are typically white in appearance early on and can later become more pigmented. Fuchs’ heterochromic iridocyclitis and herpetic uveitis can produce KPs outside of Arlt’s triangle.
Band keratopathy may also be present in chronic uveitis. Non-granulomatous disease will manifest small white KPs, while larger, “mutton-fat” KPs are more prevalent in granulomatous uveitis. The granulomatous distinction may be helpful, because a select number of diseases are classified as such. They include TB, cat-scratch disease, sarcoidosis, syphilis, Lyme disease and some cases of herpetic uveitis.
• Anterior chamber. In the anterior chamber, cells (leukocytes) and flare (protein) can appear
in the aqueous humor. In severe cases, fibrin or a hypopyon may be present.
Persistent flare, which does not resolve with treatment, can be seen in long-standing uveitis. The presence of flare may predispose a patient to synechiae, which can lead to angle-closure glaucoma if pupillary block develops.
Within the iris, sectoral atrophy may be present in herpetic disease. In granulomatous disease, the iris may display nodules at the pupillary margin (Koeppe), within the stroma (Busacca) or in the angle (Berlin).
• Lens. Cataracts can occur either from longstanding inflammation or prolonged use of corticosteroids. Usually, they are of the posterior subcapsular type, but they may also be cortical.
• Posterior segment. The retina, optic nerve and choroid can display varying signs of inflammation. The optic nerve head may develop edema. The vessels may display vascular sheathing or hemorrhages, and retinal edema or necrosis will cause whitening.
 |
Atrophy can develop after inflammation resolves and may predispose the eye to retinal breaks and detachments. Deep to the retina, creamy yellow lesions can represent choroidal infiltration. If Bruch’s membrane is broken, choroidal neovascularization may develop.
Within the vitreous cavity, vitritis, snowballs (especially suggestive of sarcoidosis and intermediate uveitis),18 fibrosis or cyclitic membranes may be present. The location of white cells in the vitreous can help pinpoint the source of inflammation. In iridocyclitis, cells are located more anteriorly, while in intermediate or posterior uveitis, cells are more distributed or appear posteriorly. Snowbanking overlying the ora serrata can occasionally occur.
Differential Diagnosis
Classification can help to rapidly narrow the differential diagnosis. While much overlap occurs, many etiologies are strongly linked to certain uveitis classes. For example, HLA-B27 positivity is characteristic of acute anterior uveitis, whereas toxoplasmosis is associated with a posterior uveitis. Along with the classification of uveitis, other important clues to the differential include ethnicity and exposure history. Table 1 provides a breakdown of classifications and their associated differentials with systemic findings.
Lab Testing and Imaging
• Laboratory testing. Tailor lab testing to the clinical situation. Most cases of unilateral, nongranulomatous anterior uveitis do not need a laboratory workup. More complex situations may require testing tailored to the patient. Table 3 lists recommended laboratory tests for the different suspected etiologies of uveitis.
The most appropriate way to approach laboratory testing is to form a differential diagnosis and then customize the testing accordingly. However, nearly every lab panel for uveitis should include testing for syphilis, sarcoidosis and TB.16
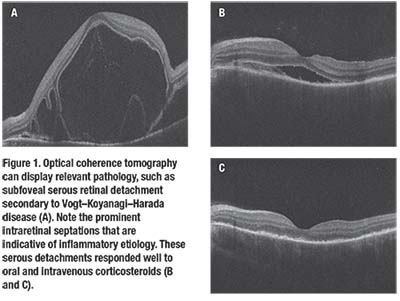
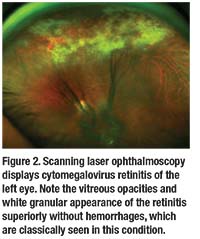
• Imaging. Testing can also include optical coherence tomography to evaluate for cystoid macular edema and choroidal thickness if enhanced depth imaging is available (Figure 1); fluorescein angiography to assess for vascular leakage and serous retinal detachments; and ultrasonography to evaluate for posterior scleritis. Another option is scanning laser ophthalmoscopy (Figure 2).
Systemic imaging, such as chest X-ray and CT scan, can be helpful when sarcoidosis is suspected. In cases unresponsive to treatment or in which infectious or neoplastic causes are suspected, diagnostic vitrectomy or chorioretinal biopsy may help increase diagnostic certainty.
Treatment for Uveitis
The primary goal of treatment in uveitis is to
 |
reduce inflammation and preserve sight. This often requires either antimicrobial or immunomodulatory agents.
• Corticosteroids. These agents are first-line therapy in most cases of noninfectious uveitis and are especially helpful in anterior uveitis.
The rule of thumb is to use the safest, most potent agent (Table 4, page 30). Most clinicians start with prednisolone acetate. Assess IOPs in any patient on topical steroids at least two weeks after beginning the steroid. Monitor any patient with a history of steroid response closely.
• Alternatives to topical steroids. For patients that cannot tolerate topical steroids, triamcinolone can be injected into the sub-Tenon’s space. This can be given every month and titrated to patient response. Triamcinolone can also be administered intravitreally and should last weeks to months.
Another intravitreal option is the dexamethasone degradable implant (Ozurdex, Allergan), which lasts one to three months. A fluorocinolone acetonide-sustained release device (Retisert, Bausch + Lomb) can be sutured at the pars plana; it lasts 2.5 to three years.
In cases that involve anterior inflammation, a cycloplegic will also prevent ciliary spasm and help ameliorate photophobia. In addition, movement and deepening of the iris help to prevent the formation of posterior and anterior synechiae.
• Systemic corticosteroids. Severe uveitis of any location or posterior uveitis may warrant oral or intravenous c
 |
orticosteroids. The typical dose of oral prednisone starts at 1 mg/kg (maximum 60 mg/day), while intravenous methylprednisolone starts at 1 gr/day for three days and is then followed with an oral prednisone taper.
Any patient on more than two weeks of systemic steroids requires a taper. Patients should never stay on a dose greater than 7.5 to 10 mg per day chronically. Patients on long-term therapy should also receive calcium and vitamin D supplementation.
• Immunomodulatory agents. If corticosteroids do not achieve control, immunomodulatory agents are an option. Locally, sirolimus has shown promise as a bimonthly intravitreal therapy. Phase I data showed good control, and the SAVE trial showed it reduced vitreous haze and the need for corticosteroid therapy. The SAKURA study demonstrated that sirolimus
reduced ocular inflammation and increased the probability of tapering steroids.19–21
Systemic Therapy
If local immunomodulatory control is inadequate, the patient may need systemic treatment. Clinicians who are not comfortable using these medications should refer the patient to a uveitis specialist or rheumatologist. Limited data exist, and most providers will base medication choice on familiarity, side-effect profile and comorbidities.
• Antimetabolites. These agents include methotrexate, mycophenolate and azathioprine. If greater control is needed, another antimetabolite or drug from a new class can be added. Adalimumab (Humira, Abbvie), a biologic tumor necrosis factor-alpha inhibitor, is approved for noninfectious intermediate, posterior and panuveitis.22
• Alkylating agents. If a combination of classes cannot control the disease, an alkylating agent may be needed. This class carries significant side effects, namely the risk of sterility, bone marrow suppression and increased risk of malignancy.
Visual Prognosis
The visual prognosis
 |
for uveitis varies widely based on location. The chances of a 25-percent decrease in visual acuity, based on location, are as follows:23
-anterior uveitis, 1 to 4 percent;
-posterior uveitis, 43 percent;
-intermediate uveitis, 66 percent; and
-panuveitis, 40 percent.
CME followed by cataract and glaucoma causes visual impairment or blindness in up to 35 percent of patients.9,24
Despite which specialist manages the medical therapy, the ophthalmologist plays a vital role in monitoring these patients and treating ocular complications such as ocular hypertension, CME, retinal detachments, hypotony and cyclitic membranes.
Clinicians who carefully consider the presenting signs and symptoms of uveitis, examination findings, differential diagnosis and treatment options are well-positioned to adequately manage the disease and preserve sight.
Even with good control, both uveitis patients and clinicians must remain watchful for recurrences to prevent irreversible damage. Due to the expanding armamentarium of therapeutic options, many uveitis patients can achieve excellent control of inflammation and have minimal ocular and systemic side effects. RS
REFERENCES
1. Nussenblatt RB. The natural history of uveitis. Int Ophthalmol. 1990;14:303-308.
2. Darrell RW. Epidemiology of uveitis. Arch Ophthalmol. 1962;68:502.
3. Reeves S, Sloan F, Lee P, Jaffe G. Uveitis in the elderly: Epidemiological data from the National Long-term Care Survey Medicare Cohort. Ophthalmology. 2006;113:302–307.
4. Gritz D. Incidence and prevalence of uveitis in Northern California: The Northern California Epidemiology of Uveitis Study. Ophthalmology. 2004;111:491-500.
5. Acharya NR, Tham VM, Esterberg E, et al. Incidence and prevalence of uveitis. JAMA Ophthalmology. 2013;131:1405.
6. Suhler EB, Lloyd MJ, Choi D, Rosenbaum JT, Austin DF. Incidence and prevalence of uveitis in Veterans Affairs Medical Centers of the Pacific Northwest. Am J Ophthalmol. 2008;146:890-896.
7. Thorne JE, Suhler E, Skup M, et al. Prevalence of noninfectious uveitis in the United States. JAMA Ophthalmology. 2016;134:1237.
8. McCannel CA, Holland GN, Helm CJ, et al. Causes of uveitis in the general practice of ophthalmology. Am J Ophthalmol. 1996;121:35-46.
9. Suttorp-Schulten MS, Rothova A. The possible impact of uveitis in blindness: A literature survey. Br J Ophthalmol. 1996;80:844-848.
10. Jabs DA, Nussenblatt RB, Rosenbaum JT. Standardization of Uveitis Nomenclature Working Group. Standardization of uveitis nomenclature for reporting clinical data. Results of the First International Workshop. Am J Ophthalmol. 2005;140:509-516.
11. Päivönsalo-Hietanen T, Vaahtoranta-Lehtonen H, Tuominen J, Saari KM. Uveitis survey at the University Eye Clinic in Turku. Acta Ophthalmologica. 2009;72:505-512.
12. Rodriguez A. Referral patterns of uveitis in a tertiary eye care center. Arch Ophthalmol. 1996;114:593.
13. Henderly DE, Genstler AJ, Smith RE, Rao NA. Changing patterns of uveitis. Am J Ophthalmol. 1987;103:131-136.
14. Tsirouki T, Dastiridou A, Symeonidis C, et al. A focus on the epidemiology of uveitis. Ocular Immun Inflam. 2016;26:2-16.
15. Pratas AC, Goldschmidt P, Lebeaux D, et al. Increase in ocular syphilis cases at ophthalmologic reference center, France, 2012-2015. Emerging Infect Dis. 2018;24:193-200.
16. Read RW. General approach to the uveitis patient and treatment strategies. In: Yanoff M, Duker JS, eds. Ophthalmology. 4th ed. Saunders; 2014.
17. Engelhard S, Patel V, Reddy A. Intermediate uveitis, posterior uveitis, and panuveitis in the Mid-Atlantic USA. Clinical Ophthalmology. 2015;9:1549-1555. doi:10.2147/OPTH.S89428.
18. Acharya NR, Browne EN, Rao N, Mochizuki M, for International Ocular Sarcoidosis Working Group. Distinguishing features of ocular sarcoidosis in an international cohort of uveitis patients. Ophthalmology. 2018;125:119-126.
19. Nguyen QD, Merrill PT, Clark WL, et al. Intravitreal sirolimus for noninfectious uveitis: A Phase III Sirolimus Study Assessing Double-masKed Uveitis TReAtment (SAKURA). Ophthalmology. 2016;123:2413-2423.
20. Maya JR, Sadiq MA, Zapata LJ, et al. Emerging therapies for noninfectious uveitis: What may be coming to the clinics. J Ophthalmol. 2014;2014:310329–7. doi:10.1155/2014/310329.
21. Nguyen QD, Ibrahim MA, Watters A, et al. Ocular tolerability and efficacy of intravitreal and subconjunctival injections of sirolimus in patients with non-infectious uveitis: Primary 6-month results of the SAVE Study. J Ophthalm Inflam Infec. 2013;3:32. 22. Díaz-Llopis M, Salom D, Garcia-de-Vicuña C, et al. Treatment of refractory uveitis with adalimumab: A prospective multicenter study of 131 patients. Ophthalmology. 2012;119:1575-1581.
23. Linssen A, Meenken C. Outcomes of HLA-B27-Positive and HLA-B27-negative acute anterior uveitis. Am J Ophthalmol. 1995;120:351-361.
24. Rothova A, Suttorp-van Schulten MS, Frits Treffers W, Kijlstra A. Causes and frequency of blindness in patients with intraocular inflammatory disease. Br J Ophthalmol. 1996;80:332-336.



