 |
Shortly thereafter, ophthalmologists started using systemic corticosteroids for the management of uveitis, and several reported cases were published in the literature in 1950.5-7 Corticosteroid drops and local steroid injections were subsequently developed and utilized. These developments dramatically changed the landscape for treatment of noninfectious uveitis.
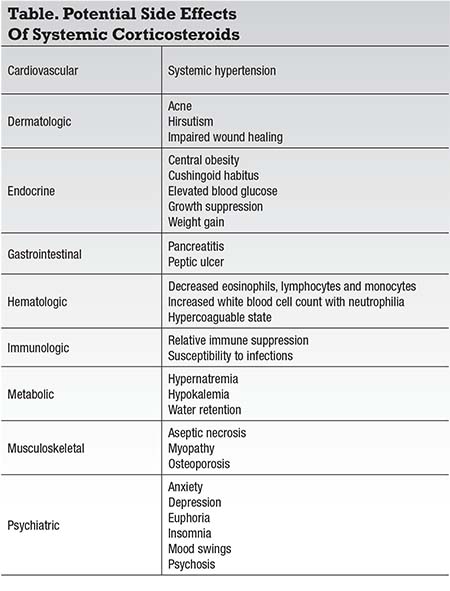
Despite the clinical efficacy of systemic and local corticosteroids in the management of uveitis, significant side effects have always been the main limitation for their use. Systemic corticosteroids have well known cardiovascular, dermatologic, endocrine, gastrointestinal, hematologic, immunologic, metabolic, musculoskeletal
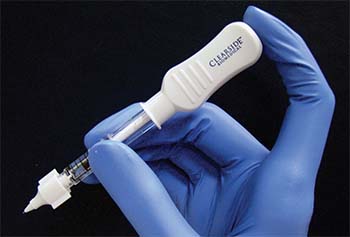 |
| Figure 1. The Clearside Biomedical CLS-TA microinjector uses a leur-lock attachable 30-gauge microneedle that comes in 900-μm and 1,100-μm lengths. |
Ophthalmic side effects of systemic and local corticosteroids are all too familiar to ophthalmologists; they include cataract formation, elevated intraocular pressure, central serous chorioretinopathy and susceptibility to opportunistic ocular infections (amoeba, bacterial, fungal, parasitic and viral), amongst others.8-9
The options for the use of both local and systemic corticosteroids continue to expand. This article discusses a new method for delivery of local steroids, via a suprachoroidal microinjector, and will also discuss the use of a subcutaneous repository corticotropin gel, an old pharmacologic agent that is re-emerging for use in ophthalmology.
Suprachoroidal Injection
It is an oft-repeated sentiment that the use of local corticosteroids cannot be separated from their frequent side effects of cataracts and elevated intraocular pressure. Clearside Biomedical is developing potential treatments for eye diseases via suprachoroidal administration. To enable this procedure, Clearside has developed CLS-TA, a proprietary microinjector syringe to deliver triamcinolone acetonide via the suprachoroidal space to mitigate these side effects.10-12
The microinjector uses a leur-lock attachable 30-gauge microneedle that comes in 900-μm and 1,100-μm lengths (Figure 1). During the procedure, the needle is held perpendicular to the sclera, approximately 4 mm posterior to the corneoscleral limbus. The needle indents the sclera slightly and enters the suprachoroidal space. Triamcinolone acetonide 4 mg/0.1 mL is then injected slowly over three to five seconds. When the needle is correctly positioned past the sclera, the medication can be infused to the suprachoroidal space without significant resistance (Figure 2).
Pre-clinical rabbit studies have suggested that concentrated amounts of the triamcinolone acetonide were delivered to the retina and choroid with minimal levels of the medication being detected in the anterior chamber, theoretically reducing the risk of elevated IOP and cataract formation or progression.11
Clinical Trials of CLS-TA
Results from a Phase I/II open-label study were recently published.10 This study enrolled nine patients over a 26-week follow-up period. A total of 11 injections were attempted, and eight injections were successful in delivering the drug to the suprachoroidal space. The mean change in IOP was +0.9mm Hg (range: -4mm Hg to +6mm Hg) at 26 weeks, and no patients required IOP-lowering medications. One patient developed a cataract, but the medication was not successfully delivered in this patient.
The most common reported side effect was transient eye pain, occurring during five of the 11 attempted injections. Two patients had a temporary reduction in vision following the injection. Through week eight, five eyes (63 percent) receiving the injection had achieved a 3-line or greater improvement in visual acuity. Mean visual acuity improvement ranged from 8 to 14 letters gained. Mean reduction in central subfield thickness was 154 μm at eight weeks (range 61-375).
A subsequent Phase II, masked, randomized,
 |
The study met its primary endpoint, with subjects in the high-dose arm demonstrating a reduction in macular edema (164 μm, p=0.0017) through two months of follow-up. These patients also showed a statistically significant mean improvement in visual acuity (9.2 letters, p=0.0004).
Eye pain was again the most common ocular adverse event, occurring in three of 17 subjects. One patient had a temporary increase in IOP at the time of injection, but no cases of sustained IOP elevations were attributable to the suprachoroidally injected steroid, and no patients required IOP-lowering drops.
A Phase III, randomized-controlled trial for patients with macular edema associated with non-infectious uveitis (PEACHTREE) is currently enrolling patients.13 Additionally, further safety data are being collected in a Phase III open-label trial (AZALEA).14
Acthar Gel
One of the original medications that Dr. Hench used in management of his rheumatology patients was the melanocortin peptide, adrenocorticotropic hormone (ACTH).15-19 Acthar gel (Mallinckrodt Pharmaceuticals) contains ACTH, which acts on the adrenal glands to stimulate the endogenous production of glucocorticoids. The medication also has steroid-independent properties mediated through its binding to five different melanocortin receptors, which are expressed on T-helper cells, T-regulatory cells, macrophages and dendritic cells.
The melanocortin receptors are also expressed on a number of individual tissues throughout the eye and central nervous system, including retinal ganglion cells and retinal pigment epithelium cells.19
The medication is administered subcutaneously or intramuscularly at a dosage of 40 units/0.5 mL or 80 units/1 mL, typically twice weekly. Acthar has been Food and Drug Administration (FDA)-approved for the treatment of anterior uveitis, posterior uveitis and optic neuritis since 1952, but its use has only recently increased.15-22
In rheumatology, Acthar has been used mostly as adjunctive therapy in the management of ankylosing spondylitis, dermatomyositis, polymyositis, psoriatic arthritis, sarcoidosis and systemic lupus erythematosus.16 Neurologists also use Acthar as a primary therapy for infantile spasm as well as acute flares in multiple sclerosis.16
A recent retrospective cohort
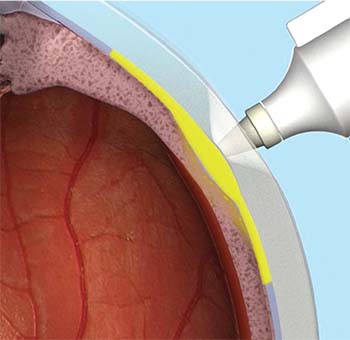 |
| Figure 2. The CLS-TA microinjector needle is held perpendicular to the sclera, approximately 4 mm posterior to the corneoscleral limbus. When properly positioned, the needle can infuse the medication into the suprachoroidal space with minimal resistance. |
Among the patients completing treatment, two had an improvement in uveitis and one remained stable with a reduction in dosage of oral corticosteroids.
Despite Acthar having FDA-approval for the management of uveitis, little has been published about its clinical efficacy for its treatment.21,22 A case report recently described the successful use of Acthar in a 33-year-old man with unilateral idiopathic panuveitis and retinal vasculitis.21 The patient was treated with twice-weekly subcutaneous ACTH gel injections over a six weeks; his
 |
Another report involved the use of Acthar in a 16-year-old girl with a history of refractory uveitis secondary to juvenile idiopathic arthritis.22 Despite ongoing therapy with methotrexate, infliximab, topical corticosteroids and chronic low-dose oral steroids, the patient continued to have active iridocyclitis and sclerokeratitis. After a trial of toclizumab failed to control inflammation, Acthar 80 units/1 mL twice weekly intramuscularly was added to her treatment regimen.
Within two weeks, the patient’s visual acuity had improved from 20/400 to 20/60 in the right eye and from 20/100 to 20/50 in the left eye. At six months, visual acuity had improved to 20/25 in the right eye and 20/20 in the left eye, with complete resolution of iridocyclitis and sclerokeratitis and a reduction in corneal edema. The medication is currently in Phase IV studies as well as investigator-sponsored trials, with results forthcoming.
What’s Ahead
Corticosteroids will continue to have an important role in the management of patients with uveitis, but their traditional use has been limited by predictable and significant side effects. Forthcoming data will reveal whether the suprachoroidal delivery of steroids can prove efficacious, while limiting local side effects. Further studies on Acthar gel may help determine if this medication should re-emerge as a worthy therapeutic option for patients with uveitis. RS
REFERENCES
1. Hench PS, Kendall EC, Slocumb CH, Polley HF. The effect of a hormone of the adrenal cortex (17-hydroxy-11-dehydrocorticosterone: compound E) and of adrenocorticotropic hormone on rheumatoid arthritis. Proc Staff Meet Mayo Clin. .1949:24:181-197.
2. Hench PS. The ameliorating effects of pregnancy on chronic atrophic (infectious rheumatoid) arthritis and intermittent hydroarthrosis. Proc Staff Meet Mayo Clin. 1938;13:157-172.
3. Benedek T. History of the development of corticosteroids. Clin Exp Rheumatol 2011;29:suppl 68.
4. Kirwan JR, Balint G, Szebenyi B. Anniversary: 50 years of glucocorticoid treatment in rheumatoid arthritis. Rheumatology (Oxford). 1999;38:100-102.
5. Theodore FH. Use of cortisone in severe bilateral uveitis. Eye Ear Nose Throat Mon. 1950;29:314-315.
6. Posner A. Cortisone in the treatment of recurrent iritis associated with Marie-Struempell spondylitis. Eye Ear Nose Throat Mon. 1950;29:316-317
7. Henderson JW. Clinical observations on the use of cortisone in ophthalmic disease: preliminary report. Proc Staff Meet Mayo Clin. 1950;25:459-462.
8. Stanbury RM, Graham EM. Systemic corticosteroids therapy—side effects and their management. Br J Ophthalmol. 1998:82:704-708
9. Becker B, Mills DW. Elevated intraocular pressure following corticosteroid eye drops. JAMA. 1963;185:884-886.
10. Goldstein DA, Do D, Noronha G, Kissner JM, Srivastava SK, Nguyen QD. Suprachoroidal corticosteroid administration: a novel route for local treatment of noninfectious uveitis. Trans Vis Sci Tech. 2016;5:14.
11. Edelhauser HF, Verhoeven RS, Burke B, Struble CB, Patel SR. Intraocular distribution and targeting of triamcinolone acetonide suspension administered into the suprachoroidal space. Invest Ophthalmol Vis Sci. 2014;55:5259.
12. Yeh S. Suprachoroidal administration of triamcinolone acetonide: results of a phase 2 study of patients with noninfectious uveitis. Paper presented at American Uveitis Society Winter Symposium; January 14, 2016; Park City, UT.
13. Clearside Biomedical. Suprachoroidal injection of CLS-TA in subjects with macular edema associated with non-infectious uveitis (PEACHTREE). NLM identifier: NCT02595398.Available from: https://clinicaltrials.gov/ct2/show/NCT02595398 Accessed May 25, 2017.
14. Suprachoroidal injection of CLS-TA in subjects with non-infectious uveitis. ClinicalTrials.gov. https://clinicaltrials.gov/ct2/show/NCT03097315. Accessed May 25, 2017
15. Montero-Melendez T. ACTH: the forgotten therapy. Semin Immunol. 2015;27:216-226.
16. H.P Acthar Gel [package insert]. Hazelwood, MO; Mallinckrodt ARD Inc.
17. Clemson CM, Yost J, Taylor AW. The role of alpha-MSH as a modulator of ocular immunobiology exemplifies mechanistic differences between melanocotins and steroids. Ocular Immunol Inflamm. 2017;25:179-189.
18. Catania A, Lonati C, Sordi A, Carlin A, Leonardi P, Gatti S. The melanocortin system in control of inflammation. Sci World J. 2010;10:1840-1853.
19. Taylor AW, Lee D. Applications of the role of α-MSH in ocular immune privilege. Adv Exp Med Biol. 2010;681:143-149.
20. Baughman RP, Barney JB, O’Hare L, Lower EE. A retrospective pilot study examining the use of Acthar gel in sarcoidosis patients. Respir Med. 2016;110:66-72.
21. Agarwal A, Hassan M, Sepah YJ, Do DV, Nguyen QD. Subcutaneous repository corticotropin gel for non-infectious panuveitis: reappraisal of an old pharmacologic agent. Am J Ophthalmol Case Reports. 2016;4:78-82.
22. Ahmad KT, Sukumaran S, Brown KL, Ali TK. Use of corticotropin hormone for treatment of refractory uveitis secondary to juvenile idiopathic arthritis. Poster presentation at the 9th International Symposium on Uveitis; August 20, 2016; Dublin, Ireland.



