In a giant retinal tear (GRT), the vitreous is firmly attached to the anterior edge of the tear while the posterior flap is free from any vitreous attachment, allowing it to scroll and roll toward the posterior pole. The presence of posterior vitreous detachment (PVD) further increases the propensity for flap mobility and subsequent tear inversion.1
RRD related to GRT has always been a surgical challenge for vitreoretinal surgeons because of the increased risk of retinal slippage, redetachment and proliferative vitreoretinopathy (PVR). Over the years, however, authors have described several approaches that can improve outcomes. Here, we present an up-to-date review of GRT with RRD, looking at the common issues surgeons encounter and some of the current thinking about management.
 |
Vitrectomy
The use of preservative-free triamcinolone (Triesence, Alcon) is helpful in confirming intraoperative PVD and performing a complete vitrectomy. Take care to ensure that all vitreous attachments are released from the posterior and anterior flap tear. Under scleral indentation, perform a meticulous 360° shaving of the vitreous base anteriorly. Trim the anterior flap of the GRT entirely to prevent anterior peripheral traction, PVR and possibly peripheral ischemia.
Rolls and Folds
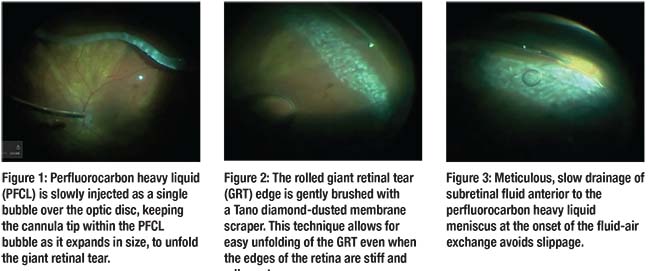
Perfluorocarbon heavy liquid (PFCL) is very useful for unfolding the posterior retinal flap in a GRT RRD. Gradually inject the PFCL as a single bubble over the optic disc, keeping the cannula tip within the PFCL bubble as it expands in size (Figure 1).
Inject the PFCL until it fills to the posterior edge of the GRT, allowing the GRT flap to unfold. It is important to inject the PFCL slowly under low infusion pressure to avoid too much turbulence and secondary multiple PFCL bubbles. We typically turn the infusion down to 5 to 10 mmHg and also use a dual-bore cannula (MedOne Surgical) to avoid this complication.
In cases of a persistent inverted or rolled GRT, you can use the soft-tip PFCL injection cannula to unfold the scrolled retina, but this may inadvertently lift the GRT edge and lead to subretinal PFCL. We’ve found that gently brushing the folded GRT with the Tano diamond-dusted membrane scraper avoids lifting of the tear and lowers the chance of inducing subretinal PFCL (Figure 2).
Laser
We routinely apply two confluent rows of laser in a continuous mode along the posterior edge of the GRT followed by three rows of scattered laser posterior to these. Pay special attention to treating the horns of the GRT with broader laser up to the ora serrata to prevent any guttering of subretinal fluid and recurrent RRD postoperatively.
An illuminated endolaser probe is helpful for ensuring that all edges of the tears are treated. We often proceed with 360° endolaser photocoagulation in two or three rows posterior to the ora serrata in cases of GRT and RRD.2
Retinal Slippage
Slippage occurs with incomplete drainage of subretinal fluid posterior to the GRT, causing reattached retina and the GRT to slide toward the posterior pole. To prevent aqueous entry into the subretinal space during this process with subsequent retinal slippage, we proceed with a slow air-fluid exchange in a stepwise anteroposterior direction.
Before aspirating PFCL, first meticulously and slowly drain all subretinal fluid anterior to the PFCL meniscus (Figure 3). Perform an air-fluid exchange by placing the tip of the extrusion cannula anterior to the GRT above the PFCL meniscus to ensure complete drainage of subretinal fluid.
Direct PFCL-to-silicone oil (SO) exchange can also reduce the likelihood of retinal slippage because of the hydrophobic nature of PFCL and SO, expelling any subretinal fluid at their interface.
When performing this technique, detach the irrigation line and replace it with a SO infusion cannula. An assistant may also hold the infusing SO cannula while it is being injected. Remove the PFCL passively through a soft-tip cannula at the optic nerve head. As the SO begins to enter the eye, removing the infusion fluid anterior to the PFCL bubble is helpful. Once this is done, you can place the cannula posteriorly over the optic nerve to remove PFCL as the SO is injected.
Tamponade Agent
Several factors influence the choice of tamponade agent in the treatment of GRT. A long-acting gas mixture of perfluoropropane (C3F8) is certainly convenient, as the gas bubble will resorb spontaneously. However, A.M. Al-Khairi, MD, and colleagues published a retrospective study of 115 patients, reporting a substantially higher rate of recurrent retinal detachment when gas was used (32 percent) than in eyes with silicone oil tamponade (13 percent).3
Nonetheless, a randomized controlled study of 47 eyes with comparable baseline characteristics showed no statistically significant differences in outcomes at 48 months postoperatively with either C3F8 or SO tamponade.4 Retinal attachment was accomplished in 86 percent of the C3F8 group, in 89 percent (eight eyes) of the retained-SO group and in 94 percent (15 eyes) of those with SO removed.
SO is undoubtedly helpful in the presence of PVR, inferior GRT or when a patient is unable to comply with postoperative positioning requirements. Studies investigating the management of GRT RRD with silicone oil concluded that the primary retinal attachment rate was between 74 percent and 96 percent.5-7
Nurten Unlu, MD, and colleagues removed the SO in all patients and, after a mean follow-up of eight months, reported a reattachment rate of 81 percent.6 Our preference is to use SO unless the tear is localized to the superior retina and PVR is absent, in which case, we would use C3F8 gas.
Scleral Buckle
The role of adjuvant encircling scleral buckling (SB) to pars plana vitrectomy (PPV) in GRT RRD is controversial among retinal surgeons. SB provides support to the vitreous base and thus relieves vitreoretinal tractional forces responsible for reopening or extension of the retinal break.8
SB, however, can result in fish-mouthing and redundant retinal folds, enabling posterior tear slippage. In our experience, we do not routinely add a scleral buckle and, in cases of an inferior GRT, we feel that heavy silicone oil (Densiron 68) with upright supine positioning achieves good results.
Proliferative Vitreoretinopathy
The rate of PVR in GRT with RRD is higher, in part, due to the extensive zone of exposed retinal pigment epithelium. Its incidence in the literature varies from 9 to 62 percent.1 To reduce PVR, we routinely inject 0.5 mg of preservative-free triamcinolone (40 mg/mL) into the vitreous cavity at the end of our surgery.
Interestingly, new medications on the horizon may eventually aid the treatment of PVR in patients undergoing GRT RRD repair. Reports have shown that resveratrol, for example, has suppressed the development of experimental PVR in rabbit eyes.9 Cannabinoids acting at the cannabinoid 2 receptor (CB2R) could also be a potential therapy for PVR. In fact, results of a study using a CB2R agonist at early stages of PVR in mouse eyes showed diminished ocular inflammation and disease severity.10
Take-Home Point
The principles of successful management of GRT with RRD consists of a thorough vitrectomy, unfolding of the posterior retinal flap tear, complete removal of subretinal fluid, creation of a firm chorioretinal adhesion around GRT and adequate internal tamponade.
Meticulous subretinal fluid drainage using the extrusion cannula at the posterior edge of the GRT is paramount to the success of GRT management. Surgical approaches, such as using adjunctive SB and selecting the type of endotamponade agent, are often individualized based on the clinical scenario and surgeon preference. RS
Dr. Mandelcorn is an assistant professor of ophthalmology at the University of Toronto. Dr. Trussart is a vitreoretinal fellow at the University of Toronto and Dr. Yan is a vitreoretinal surgeon and faculty member there.
REFERENCES
1. Shunmugam M, Ang GS, Lois N. Giant Retinal Tears. Surv Ophthalmol. 2014;59:192-216.
2. Dabour SA. The outcome of surgical management for giant retinal tear more than 180°. BMC Ophthalmology. 2014;14:86.
3. Al-Khairi AM, Al-Kahtani E, Kangave D, et al. Prognostic factors associated with outcomes after giant retinal tear management using perfluorocarbon liquids. Eur J Ophthalmol. 2008;18:270-277.
4. Batman C, Cekic O. Vitrectomy with silicone oil or long-acting gas in eyes with giant retinal tears: long-term follow-up of a randomized clinical trial. Retina. 1999;19:188-192.
5. Glaser BM. Treatment of giant retinal tears combined with proliferative vitreoretinopathy. Ophthalmology. 1986;93:1193-1197.
6. Unlu N, Kocaoglan H, Acar MA, et al. The management of giant retinal tears with silicone oil. Eur J Ophthalmol. 2003;13:192-195.
7. Mathis A, Pagot V, Gazagne C, et al. Giant retinal tears. Surgical techniques and results using perfluorodecalin and silicone oil tamponade. Retina. 1992;12(3 Suppl):S7-10.
8. Gonzalez MA, Flynn HW Jr, Smiddy WE, Albini TA, Tenzel P. Surgery for retinal detachment in patients with giant retinal tear: etiologies, management strategies, and outcomes. Ophthalmic Surg Lasers Imaging Retina. 2013;44:232-237.
9. Ishikawa K, He S, Terasaki H, et al. Resveratrol inhibits epithelial-mesenchymal transition of retinal pigment epithelium and development of proliferative vitreoretinopathy. Sci Rep. 2015:10;5:16386. doi: 10.1038/srep16386.
10. Szxzesniak AM, Porter RF, Toguri JT, et al. Cannabinoid 2 receptor is a novel anti-inflammatory target in experimental proliferative vitreoretinopathy. Neuropharmacology. 2017;113(Pt B):627-638.




