Conventional thinking suggests that the destruction of tissue results in a reduced hypoxic state by lowering the demand for oxygen and decreasing the overall metabolic rate of the retina.3,4 While destructive laser photocoagulation has beneficial effects, it also causes side effects such as reduced night vision, macular and peripheral scotomas and disruption of the retinal anatomy through scarring.5 But, what if we could achieve the benefits of laser therapy without the destruction of tissue?
Changes in Gene Expression
Recent studies have shown that eyes undergoing photocoagulation over-express more than 25 genes.6 These genes represent diverse biological functions including photoreceptor metabolism, synaptic function, structural proteins, adhesion-specific proteins and proteins induced by cellular stress and increased thermal conditions known as heat shock proteins (HSPs).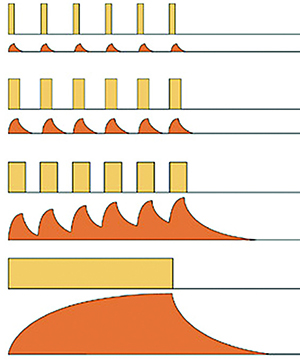 | < Low-duty cycle: Very little thermal spread can occur during the “on” time and the releatively long “off” time favors the cool-off before the arrival of the next pulse. < Medium-duty cylce: Doubling the “width” of the pulse doubles the energy deposited, increases the heat spread during the “on” time and reduces the cool-off time, but can still avoid cumulative thermal build-up. < High-duty cylce: More energy is deposited with more thermal spread during the “on” time and there is some thermal build-up due to the shorter cool-off time before the next pulse. < CW Pulse (100% duty cycle): The thermal rise and re-equilibration can only be controlled by adjusting the power and the exposure duration of the CW laser emission. | | ||
| Figure 1. This diagram illustrates the four cycles of micropulse tissue heat control. (Courtesy of Iridex) | ||||
Laser photocoagulation also decreases the expression of permeability factors, inducers of vascular endothelial growth factor (VEGF) and the levels of transforming growth factor ßII (TGF-ßII).6 Most likely, all these changes in gene expression contribute to the decrease in neovascularization and vascular permeability associated with laser photocoagulation.
The Sub-Threshold Concept
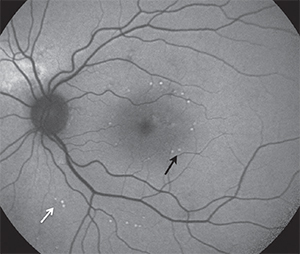
photothermal stimulation with landmarks (100 percent
energy), which appear as spots with increased FAF (black
arrows). Titration points are also visible in FAF outside the
arcades (white arrows). The 30 percent treatment spots
were not visible clinically by FAF.
(Courtesy of Daniel Lavinsky, MD, PhD)
In the 1990s Thomas Friberg, MD, FACS, developed the MicroPulse laser (Iridex) that divided the laser emission into a “train” of short, repetitive pulses that persisted for 0.1 to 0.5 seconds. The ‘‘on’’ time was the duration of each micropulse and the ‘‘off’’ time the interval between successive micropulses.3 The “off” time allowed for heat dissipation, which decreased collateral damage and confined treatment to the retinal pigment epithelium (RPE) (Figure 1). Usually an “on” time of 5 to 15 percent has been used.
In contrast to conventional continuous wave laser therapy, which delivers the same magnitude of energy throughout the entire exposure time and causes significant collateral damage, micropulse laser is considered to be safer while possibly delivering similar therapeutic benefits with improved overall retinal sensitivity and the ability to treat and retreat in close proximity to the fovea without obvious sequellae.3,9
An added theoretical benefit of sub-threshold laser is the assumption that its efficacy would improve if more RPE cells were exposed to thermal stress. As a result, most sub-threshold treatment strategies incorporate a higher density of laser spots than conventional macular laser treatments.10
Micropulse Drawbacks
While a number of studies have reported on the clinical efficacy of micropulse laser for the treatment of central serous chorioretinopathy, diabetic macula edema and vein occlusion,10-12 a major disadvantage has been the lack of reliable titration protocols to achieve reproducible sub-visible treatments. As a result, reported treatment results have varied.3 For example, if the laser settings are too low, then the treatment will be not only sub-visible but also sub-therapeutic. If the settings are too high, excessive damage to the retina is a risk, especially with the nearly confluent coverage close to the fovea.
In addition, high-density coverage of the macula with relatively small spots and long pulse duration is time- consuming, and because the treatment delivers energy without leaving an observable mark, the surgeon has to keep track of the treated and untreated areas. Otherwise, there’s the likelihood of inadvertent re-treatment to areas during a single session, which could be deleterious.
Addressing the Drawbacks
 |
| Dr. Rosenfeld is a professor at Bascom Palmer Eye Institute, University of Miami Miller School of Medicine. He has been the principal investigator and study chair for several clinical trials involving wet and dry AMD. Dr. Schaal is a retina researh fellow at Bascom Palmer. |
As in traditional micropulse laser treatment, the EpM strategy begins retinal treatment with a titration of the laser power to a minimally visible retinal burn. This pulse energy is assigned the 100 percent level on the EpM settings, and the treatment pulse energy is then defined as a percentage of this level. The laser power and pulse duration are paired so that treatment is usually performed at a 30 percent energy level compared with the initial visible burn, a level established as the highest non-damaging setting in animal studies; it should optimize the therapy to induce HSP expression while preventing thermal damage to the RPE.7
Because each spot within the high-density laser spot pattern is irradiated with a pulse shorter than 10 ms, a large number of spots can be quickly placed using a predefined pattern and a predefined setting.13 During the application, the programmed application of 100 percent energy burns at the corner spots maintains orientation of the treatment pattern so that landmarks are visible (Figure 2).
The EpM software has several advantages over traditional micropulse therapy while apparently achieving similar efficacy. These include a proper titration protocol so that the appropriate energy level is used to stimulate the RPE in every patient, along with shorter pulses and predefined patterns with retinal landmarks that make the treatment faster and more reproducible.14 RS
References
1. Photocoagulation treatment of proliferative diabetic retinopathy. Clinical application of Diabetic Retinopathy Study (DRS) findings, DRS Report Number 8. The Diabetic Retinopathy Study Research Group. Ophthalmology. 1981; 88:583-600.2. Techniques for scatter and local photocoagulation treatment of diabetic retinopathy: Early Treatment Diabetic Retinopathy Study Report no. 3. The Early Treatment Diabetic Retinopathy Study Research Group. Int Ophthalmol Clin. 1987; 27:254-264.
3. Sivaprasad S, Elagouz M, McHugh D, Shona O, Dorin G. Micropulsed diode laser therapy: Evolution and clinical applications. Surv Ophthalmol. 2010; 55:516-530.
4. Jennings PE, MacEwen CJ, Fallon TJ, Scott N, Haining WM, Belch JJ. Oxidative effects of laser photocoagulation. Free Radical Biol Med. 1991; 11:327-330.
5. Kozak I, Luttrull JK. Modern retinal laser therapy. Saudi J Ophthalmol. 2015; 29:137-146.
6. Wilson AS, Hobbs BG, Shen WY, et al. Argon laser photocoagulation-induced modification of gene expression in the retina. Invest Ophthalmol Vis Sci. 2003;44:1426-1434.
7. Sramek C, Mackanos M, Spitler R, et al. Non-damaging retinal phototherapy: dynamic range of heat shock protein expression. Invest Ophthalmol Vis Sci. 2011; 52:1780-7.
8. Ogata N, Tombran-Tink J, Jo N, Mrazek D, Matsumura M. Upregulation of pigment epithelium-derived factor after laser photocoagulation. Am J Ophthalmol. 2001; 132:427-429.
9. Vujosevic S, Bottega E, Casciano M, Pilotto E, Convento E, Midena E. Microperimetry and fundus autofluorescence in diabetic macular edema: Subthreshold micropulse diode laser versus modified early treatment diabetic retinopathy study laser photocoagulation. Retina. 2010; 30:908-916.
10. Lavinsky D, Cardillo JA, Melo LA, Jr., Dare A, Farah ME, Belfort R Jr. Randomized clinical trial evaluating mETDRS versus normal or high-density micropulse photocoagulation for diabetic macular edema. Invest Ophthalmol Vis Sci. 2011; 52:4314-4323.
11. Roisman L, Magalhaes FP, Lavinsky D, et al. Micropulse diode laser treatment for chronic central serous chorioretinopathy: a randomized pilot trial. Ophthalmic Surg Lasers Imaging Retina. 2013; 44:465-470.
12. Parodi MB, Spasse S, Iacono P, Di Stefano G, Canziani T, Ravalico G. Subthreshold grid laser treatment of macular edema secondary to branch retinal vein occlusion with micropulse infrared (810 nanometer) diode laser. Ophthalmology. 2006; 113:2237-2242.
13. Lavinsky D, Sramek C, Wang J, et al. Subvisible retinal laser therapy: titration algorithm and tissue response. Retina. 2014; 34:87-97.
14. Lavinsky D, Palanker D. Nondamaging photothermal therapy for the retina: initial clinical experience with chronic central serous retinopathy. Retina. 2015; 35:213-22.




