 |
This year, we present full citations in the references, including abstract numbers that you can use to locate the original report at arvo.org. We also note disclosures.
Is Robot Superior For ERM or ILM Peeling?
For years, many surgical disciplines outside of ophthalmology have used robotic surgery to add precision to their operations. This study from the University of Oxford provided the first glimpse of the use of a robot for retinal surgery (Figure).1
The investigators randomized 12 eyes requiring epiretinal membrane or internal limiting
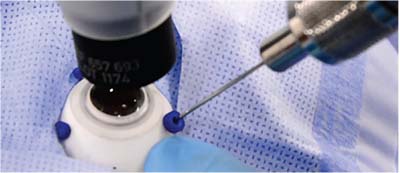 |
| Figure. Insertion of the microneedle through a port in simulated robotic ocular surgery. |
The primary comparative task was initiation of the flap for membrane peeling. In the robotic group, there were a total of two retinal hemorrhages and one incident of retinal touch with flap initiation, compared to five hemorrhages and two retinal touches in the manual group. The mean time to initiate the flap was on average 43 seconds long-er using the robot (213 ± 51 seconds vs. 130 ± 118 seconds).
This study demonstrated the first safe utilization of a robot for retinal surgery in human eyes. The robot eliminates instability from a surgeon’s microtremor and facilitates extremely precise surgical maneuvers. Given these advantages, robotic surgery may prove to be the superior option for future treatments requiring transretinal surgery with stem cells and gene therapy.
Four investigators disclosed relationships with Preceyes BV, the Eindhoven, Netherlands-based company commercializing the robotic surgery technology that has been developed at the Eindhoven University of Technology.
FA Implant for Treatment of Diabetic Retinopathy
The fluocinolone acetonide (FAc) intravitreal implant (Iluvien, Alimera Sciences) delivers continuous local therapy for 36 months. The Food and Drug Administration approved Iluvien as a treatment for diabetic macular edema after safety and efficacy were established using the three-year randomized clinical trial known as the FAME study.
A post-hoc analysis of the FAME study demonstrated that intravitreal injection
 |
Patients from the FAME trial who received 0.2 µg/day Iluvien were followed over 36 months and assessed for change in progression of proliferative diabetic retinopathy (PDR), mean Diabetic Retinopathy Severity Scale (DRSS) score and time to at least a two-step improvement or worsening in diabetic retinopathy (DR) grade compared with sham control-treated patients.
At 36 months, the proportion of patients with PDR progression was significantly reduced in Iluvien-treated patients when compared with those patients treated with sham (18 percent vs. 31 percent, respectively; p<0.001). In patients with baseline nonproliferative DR (NPDR) levels 47 to 53 (moderate-to-severe NPDR), PDR progression was 18 percent in the treatment group and 35 percent in controls (p<0.002).
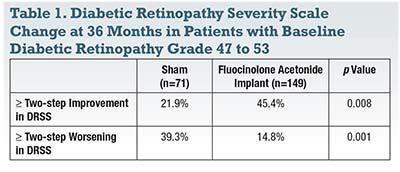
The investigators reported a statistically significant difference in change in overall mean DRSS score between Iluvien- and sham-treated patients at almost every time point during the 36-month trial. The Iluvien-treated group also had a significantly greater proportion of patients that achieved at least a two-step improvement in DRSS score and a reduced proportion of patients with a two-step or greater worsening in DRSS score over time (Table 1).
This study demonstrated that the Iluvien implant slows the progression to PDR at a similar rate to monthly anti-VEGF dosing. Furthermore, the implant improves diabetic retinopathy severity, although to a lesser degree when compared to monthly anti-VEGF dosing.
The overall benefits for diabetic retinopathy may support earlier use of Iluvien for younger pseudophakic diabetic patients to prevent progression of their retinopathy over time.
The study author disclosed a relationship with Alimera Sciences.
Topical Latanoprost to Slow Myopia Progression
Topical latanoprost, commonly used to treat glaucoma, was found to also reduce the progression of myopia in guinea pig eyes. Eight animals underwent myopia induction using monocular form deprivation. Four animals received daily topical latanoprost in their deprived eyes, while remaining control animals received topical artificial tears.3 The treatment lasted for 12 weeks.
Interocular optical axial length differences changed minimally in the latanoprost group, from -0.05±0.06 to -0.01±0.05 mm, compared to -0.01±0.04 to 0.22± 0.13 mm in the control group. Thus, the latanoprost group developed minimal myopia; interocular refractive error differences changed from -0.44±1.1 to 0.5±1.06 D compared to 0.81± 1.46 to -8.5± 0.70 D in the control group.
Current projections estimate that myopia will affect half of the world’s population by 2050, with expected rises in the rates of retinal detachments, myopic degeneration and other sight-threatening complications raising real concern. This study suggests that topical latanoprost may be an easily administered and effective topical therapy to halt the progression of myopia. Larger, controlled studies in humans will be important to validate this intriguing finding.
The authors had no pertinent disclosures.
OCT-A Detects Quiescent Neovascularization in AMD
Quiescent choroidal neovascularization (CNV) can be detected using OCT-A in nonexudative AMD.4 A multicenter, prospective consecutive case series analyzed swept-source OCT angiography (SS-OCT-A) of eyes with intermediate AMD (iAMD) or geographic atrophy (GA) secondary to nonexudative AMD in one eye and a history of exudative AMD in the fellow eye to determine the prevalence of quiescent CNV. The study also determined time to exudation and cumulative incidence of exudation in these quiescent neovascular membranes.
The researchers performed SS-OCT-A on 152 eyes (111 with iAMD and 41 with GA). Quiescent CNV was detected in 20 of the iAMD eyes (18 percent) and seven of the eyes with GA (17 percent). Eleven of 152 eyes developed exudation; 10 of those 11 eyes were previously diagnosed with quiescent CNV. The one eye developing exudation that was not diagnosed with a pre-existing neovascular lesion did not have adequate follow-up visits.
After 12 months, the cumulative incidence of exudation in all 152 eyes was 8.7 percent, and by 24 months it was 27.3 percent. In eyes with quiescent CNV, 29.7 percent of eyes developed exudation by 12 months. There was no difference in the predicted cumulative onset of exudation from pre-existing CNV in eyes with either iAMD or GA (p=0.89, log-rank test).
This study demonstrates that nearly all eyes with iAMD or GA that develop exudation over two years have a detectable quiescent CNV on SS-OCT-A prior to the event. These eyes may benefit from regular screening with SS-OCT-A for early detection of quiescent CNV. Further study is required to determine the possible benefits of treating quiescent CNV with anti-VEGF agents prior to exudation.
Several authors disclosed a relationship with Carl Zeiss Meditec.
Subretinal Transplantation of Stem Cells for GA
Subretinal transplantation of human central nervous system stem cells (HuCNS-SC) appears to be associated with a slower expansion of GA in the quadrant in which the treatment was delivered.5 A multicenter prospective clinical trial examined 22 eyes in 11 subjects with bilateral GA from AMD (Table 2). The transplanted eye was considered to be the study eye, while the fellow untreated eye was treated as a control.
The investigators infused a total of 1 x 106 HuCNS-SC in a volume of 0.02 mL
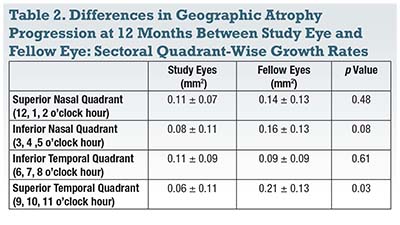 |
Twelve months after treatment, the researchers found no statistically significant difference in the mean change in total GA area between study and fellow eyes. However, the sectoral growth rate of GA in the superotemporal quadrant where the injections were placed was significantly slower in study eyes (0.06 ± 0.11 vs. 0.21 ± 0.13 mm2, p=0.03). The progression rate in the superotemporal quadrant was also significantly slower than the other three quadrants of the study eye combined (p=0.003).
The study authors concluded HuCNS-SC transplantation appears to be associated with slower growth of GA in the quadrant in which it is injected. Although further study is required, this is an exciting new development for the possible future treatment of GA, a disease for which we currently have no effective treatment. RS
One author disclosed relationships with Allergan, Genentech, Iconic, Thrombogenics, Novartis, Carl Zeiss Meditec and Optos.
REFERENCES
1. MacLaren RE, Edwards T, Xue K, et al. Results from the first use of a robot to operate inside the human eye. Invest Opthalmol Vis Sci. 2017;58:ARVO E-abstract 1185.
2. Wykoff CC. Continuous submicrogram fluocinolone acetonide (FAc) therapy for the treatment of diabetic retinopathy. Invest Opthalmol Vis Sci. 2017;58:ARVO E-abstract 2949.
3. El-Nimri N, Wildsoet CF. Effect of topical latanoprost on myopia progression in guinea pigs. Invest Opthalmol Vis Sci. 2017;58:ARVO E-abstract 5469.
4. Dias JR, Zhang Q, Roisman L, et al. Prevalence of quiescent neovascularization in intermediate and late non-exudative age-related macular degeneration and the incidence of subsequent exudation using swept-source optical coherence tomography angiography. ARVO 2017; Baltimore, MD. Program Number: 55; Poster Board Number: A0043.
5. Nittala MG, Hariri AH, Uji A, Velaga SB, Naor J, Sadda SR. Effect of human central nervous system stem cells subretinal transplantation on progression of geographic atrophy secondary to nonneovascular age-related macular degeneration. ARVO 2017; Baltimore, MD. Program Number: 29, Poster Board Number: A0017.



