 |
After each abstract, you will find a citation representing the abstract number, which you can use to locate the original report. Disclosures are also noted.
Treatment of VMT
Intravitreal injection of C3F8 gas demonstrated superior release rates for symptomatic VMT when compared to both intravitreal injections of SF6 gas and ocriplasmin (Jetrea, ThromboGenics). One hundred thirteen consecutive patients with VMT were treated with one of three interventions: 0.25 mL of 100% C3F8 gas (32 patients); 0.25 mL of 100% SF6 gas (27 patients); and 0.1 mL of ocriplasmin (54 patients). Patients who received gas injections were instructed to perform “drinking bird” head movements, in which they would bob their head forward and backward two to three times every hour for the first few days after injection.
The VMT release rate after follow-up beyond six months was 84 percent (27/32) with C3F8, 56 percent with SF6 and 48 percent with ocriplasmin. Furthermore, the patients receiving gas were characterized as having “mobile” vs. “taut” VMT by live dynamic OCT imaging during horizontal and vertical voluntary saccades.
“Mobile” VMT released more frequently than “taut” VMT with gas injection (p<0.05). No retinal breaks occurred in this series. One investigator disclosed a relationship with ThromboGenics.1806
 |
Systemic Treatment for DME
The subcutaneous injection of AKB-9778 (Aerpio Therapeutics), a Tie2 activator, in combination with intravitreal injections of 0.3-mg ranibizumab (Lucentis, Genentech), enhances reduction of DME compared to ranibizumab monotherapy. Tie2 is a receptor tyrosine kinase expressed almost exclusively in endothelial cells that becomes deactivated in diabetic patients, leading to increased vascular permeability and leakage. AKB-9778 inhibits vascular endothelial-protein tyrosine phosphatase (VE-PTP), the most critical negative downregulator of Tie2.
In this randomized, double-masked, double-dummy trial of DME patients with OCT central subfield thickness (CST) of 325 μm or greater, the participants were randomized into three groups:
• Subcutaneous AKB-9778 plus monthly sham intravitreal injection.
• Subcutaneous AKB-9778 plus monthly intravitreal 0.3-mg ranibizumab (combination group).
• Subcutaneous placebo plus monthly intravitreal 0.3-mg ranibizumab (monotherapy group).
At three months, there was a statistically significant difference in reduction of CST between the combination treatment group and the ranibizumab monotherapy group (-163.8 ±24.3 μm vs. -109.2 ±17.2 μm, p=0.008). In the combination group, fellow eyes demonstrated improvement in diabetic retinopathy severity score that was equivalent to the treatment eyes. There were no treatment group differences in adverse events.
This study demonstrated the enhanced effect of anti-VEGF therapy on DME with a well-tolerated subcutaneous medication. In addition to its benefits on the fellow eye, AKB-9778 may have further unrealized potential in the risk modification of other systemic diabetic complications related to microangiopathy, such as kidney disease. The study author disclosed a relationship with Aerpio Therapeutics.2319
 |
Dorzolamide-Timolol for AMD
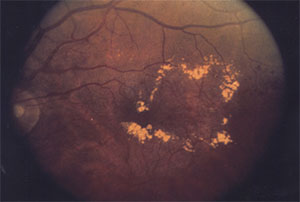
Topical dorzolamide-timolol appears to reduce subretinal fluid and CST in eyes with persistent exudation related to neovascular AMD despite consistent, fixed-interval intravitreal injections of anti-VEGF medication. This prospective study involved 10 eyes with persistent macular edema despite fixed-interval intravitreal anti-VEGF injections (mean of 21.9 prior injections). Eight eyes received aflibercept (Eylea, Regeneron Pharmaceuticals) and two were treated with ranibizumab. The enrolled patients received twice-daily topical dorzolamide-timolol in the treatment eye while continuing their previous regimen of intravitreal injections. Patients were followed for at least two visits after enrollment.
The study reported significant reduction in mean CST, from 419.7 μm at enrollment to 334.1 μm at the final visit (p = .01). Mean maximum subretinal fluid height decreased from 126.6 μm at enrollment to 49.5 μm at the final visit (p = .02). LogMAR visual acuity improved from 0.54 at enrollment to 0.48 at the final visit (p = .60).
This study suggests that topical dorzolamide-timolol may be an easily administered and effective topical adjuvant therapy for recalcitrant neovascular AMD. Larger, controlled studies will be useful to validate these intriguing findings. The authors had no relevant disclosures.4441;Poster #A0346
 |
Retinal Gene Therapy
Choroideremia is an X-linked recessive chorioretinal dystrophy caused by loss-of-function mutations in the gene CHM, generally leading to significant central vision loss in the fourth or fifth decade of life. A recent clinical trial in the United Kingdom utilizing gene therapy with adeno-associated viral (AAV) vectors for choroideremia showed sustained visual benefits in five out of six patients after 3.5 years of follow-up.
A subretinal injection of an AAV vector encoding the choroideremia gene was performed after vitrectomy in six eyes. Five eyes received a dose of 1,010 genome particles, and one eye received a dose of 6 x 109. The fellow eye was left untreated as a control in all six patients.
After 3.5 years, visual acuity change in the five eyes treated with the higher dose was +8.4 ± 4.7 letters in the treated eyes and -8.8 ± 3.1 letters in the control eyes, equivalent to a mean difference of more than three lines. The eye that received a reduced dose demonstrated a steady decline in vision over the follow-up interval.
Overall, two eyes demonstrated sustained improvements in vision, while three other eyes maintained their baseline vision despite losing vision in the fellow control eyes over 3.5 years. The encouraging outcomes from this small trial may provide a basis for gene therapy for a number of retinal diseases in the future. Several authors disclosed a relationship with Nightstarx Ltd.2297
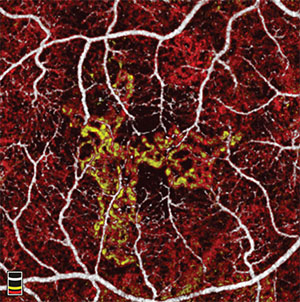 |
Choroidal neovascular (CNV) membrane area can be automatically measured with OCT angiography (RTVue-XR, Avanti, Optovue) using a saliency-based algorithm with excellent repeatability. In a small, prospective study, seven treatment-naïve patients with CNV due to neovascular AMD underwent OCT angiography scans at baseline and monthly visits while being treated on a PRN basis with anti-VEGF medication. Six of seven eyes showed initial reduction in CNV area over the course of three anti-VEGF injections, demonstrating its utility in monitoring treatment response.
In one case, CNV area decreased with treatments, then remained stable without treatment after resolution of subretinal fluid. Another patient was noted to have a reduction in CNV area and complete resolution after three monthly anti-VEGF treatments.
However, after three months of observation, the CNV area increased without the presence of fluid on OCT. One month later, the eye developed subretinal fluid requiring treatment. This suggests that CNV growth on OCT angiography may precede the recurrence of fluid on structural OCT.
The measurement of CNV area using OCT angiography provides potentially useful clinical applications of this new imaging modality in patients with CNV. Although further study is needed, the increase in CNV size may serve as an earlier indicator of the need for treatment in these patients. Several authors disclosed relationships with Optovue.2162;Poster #D0008 RS



