| ABOUT THE AUTHORS | |
 | Dr. Maldonado is a resident at Duke University Eye Center, Durham, N.C. |
 | Dr. Humayun is the Cornelius J. Pings Chair in Biomedical Sciences; professor of ophthalmology, biomedical engineering, and cell and neurobiology; director, Institute for Biomedical Therapeutics; and co-director, University of Southern California Eye Institute, Los Angeles. |
 | Dr. Hahn is assistant professor of ophthalmology for vitreoretinal surgery and diseases at Duke University Eye Center. |
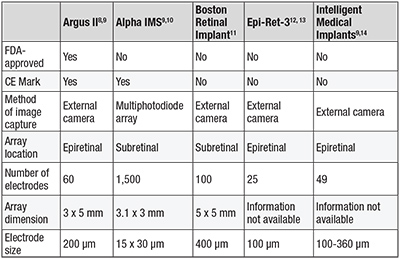
Retinal implants can be classified according to their location as epiretinal (tacked to the retinal surface) or subretinal (between photoreceptors and RPE). The Argus II epiretinal prosthesis (Second Sight Medical Products) is currently the only retinal prosthesis approved by the Food and Drug Administration and Health Canada. In Europe, the Argus II, as well as the Alpha IMS (Retinal Implant AG), a light-sensitive subretinal implant, have received the CE mark.
Other retinal implants still in development or clinical trials include the Boston Retinal Implant (Boston Retinal Implant Project), the Epi- Ret-3 (RWTH Aachen University, Germany) and Intelligent Retinal Implant System (IRIS) (IMI Intelligent Medical Implants GmbH, Germany) (Table 1, page 26).
Several alternative implant-based approaches that aim to restore vision are also in early development phases. These include cortical prostheses that directly stimulate the occipital cortex, neurotransmitter-based retinal prostheses and photovoltaic cellular approaches.4-6 In addition sensory-substitution devices, such as auditory, tactile and tongue-stimulating devices, are also undergoing investigation.7
The Argus II Retinal Prosthesis
The Argus II prosthesis is currently the only retinal prosthesis with both FDA and CE approval.9 This 60-electrode implant is a second-generation device following the Argus I, a 16-electrode implant that was placed in six subjects on an investigative basis at the University of Southern California starting in 2002 (Table 2).
Table 1. Characteristics of current retinal implants. |
 |
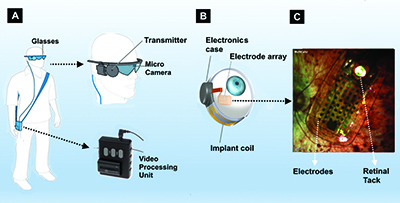
The implanted component consists of a receiving coil and electronics case secured to the eye in a scleral buckle fashion and the 60-electrode array secured to the retina with a retinal tack (Figure 1B).
The data the VPU sends wirelessly to the electronics package stimulates the array to emit small pulses of electricity that excite the remaining viable inner retina cells, including ganglion cells. These artificially stimulated retinal ganglion cells transmit signals through their axons to the lateral geniculate nucleus and then the occipital cortex, which perceives patterns of light. The final step is for patients to piece together these patterns of light into form vision.
Clinical Trials
In 2009, the Argus II was FDA-approved as a Humanitarian Use Device intended for treatment of a small patient populations (fewer than 4,000 individuals per year in the United States). To attain this designation, the Argus II was required to demonstrate safety and, on a reasonable basis, probable benefit to health that outweighs the risk of injury or illness.
In the largest prospective, single arm, unmasked, multicenter Argus II study, 30 subjects from 10 medical centers (six in the United State and four in Europe) were implanted with the Argus II on a research basis prior to FDA-approval.8 Mean age was 58 years (range of 28-77 years). Nine women and 21 men participated. Twenty-nine had a diagnosis of retinitis pigmentosa, and one had a diagnosis of choroideremia. Twenty-nine had bare light perception vision, and one had no light perception. Subjects served as their own control (system on versus off).
Because the profound vision loss in this patient population precluded accurate assessment of visual acuities by conventional methods, such as visual acuity charts, custom end-points were carefully designed in this trial.
 |
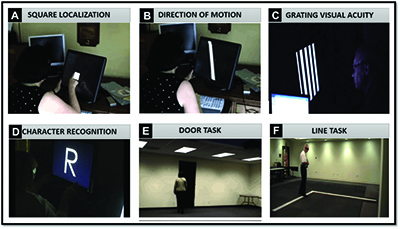
In a subsequent study of 21 Argus II subjects, patients were also asked to identify white letters (both individual and of small words) projected over a black background (Figure 2D).15
Functional vision was evaluated with a “door task” (Figure 2E) to assess the patient’s ability to find a large piece of black felt hung on a white wall to simulate a doorway and a “line task” (Figure 2F) to assess the patient’s ability to follow a white line on the floor to simulate the straight lines of a crosswalk or sidewalk.
Additionally, a Functional Low Vision Observer Rated Assessment (FLORA) was performed, in which an independent low vision specialist was asked to observe and create a narrative outlining the patient’s use of the device in his/her own home environment.
At up to three years of follow-up, investigators noted improved performance with the system on versus off in square localization, the direction of motion assessment and grating visual acuity. Interestingly, 27 percent of patients regained a measurable visual acuity (grating VA of 2.9 logMAR) with the greatest logMAR of 1.8 (equivalent to 20/1262).8 Some subjects could also identify letters, even up to a reduced size of 0.9 cm, and a few could correctly identify two, three and four letter words.15
 |
In the FLORA assessment, an independent observer noted that 77 percent of subjects had a positive effect from the Argus II, while 23 percent had no positive effect (although no patient had a negative effect). In this trial, safety data showed no permanent impairment, no loss of residual native vision and no mechanical failure of the Argus II device.8
Surgery-associated adverse events were noted in nine of 30 patients and included conjunctival erosions, endophthalmitis and hypotony. All events were treated successfully except in one patient with conjunctival erosion resulting in explantation of the Argus II.8 Many of these complications were attributed to early experiences with the Argus II, and following refinement of the implantation procedure and device itself, an improved adverse event profile has been reported.16
The ‘Right Patient’
The Argus II aims to restore rudimentary functional vision to patients with profound vision loss. It is FDA-approved for adults (age 25 years or older) with a diagnosis of retinitis pigmentosa and a history of prior useful vision who have progressed to bare light perception or no light perception vision in both eyes (Table 3).
Table 2. Brief History of the Argus II |
• 2002: First Argus I device was implanted at University of Southern California. |
Finally, the implant is not recommended in patients with a tendency to eye rubbing or with a metallic or active implantable device in the head such as cochlear implant.
Surgical Technique and Postoperative Follow-up
The implantation procedure for the Argus II was refined to require a combination of skills, many of which are familiar to the trained vitreoretinal surgeon (Table 4). The implant is secured to the wall of the eye in a fashion similar to a scleral buckle. The array is then inserted through a 5.5-mm sclerotomy and secured over the macula in a unique technique involving the use of a retinal tack to secure the implant to the retina-choroid-sclera. Care around the delicate electronics is paramount, and precisely measured external fixation of the implant is critical to allow optimal centering of the array over the macula. A patch graft is used to cover the electronics case, and suture tabs and complete closure of the overlying conjunctiva and tenon’s are important.
Table 3. Argus II Current Eligibility Criteria |
| • Adults, age 25 years or older. • Bare light or no light perception in both eyes due to retinitis pigmentosa. • Previous history of useful form vision • Aphakia or pseudophakia (if present, the natural lens will be removed during the implant procedure). The Argus II implant is intended to be implanted in a single eye, typically the worse-seeing eye. |
Within a few weeks following surgical implantation, the patient’s video processing unit (VPU) is customized to optimize each electrode according to the patient’s residual thresholds of stimulation. The VPU can be programmed with multiple, easily adjustable settings to optimize edge detection or maximize visualization of high contrast items, for example. This fitting process occurs over several days, after which the external glasses are turned on to stimulate the implanted array for the first time.
Following programming, the patient undergoes ongoing low vision rehabilitation and orientation and mobility training in conjunction with a low vision specialist and occupational therapist to learn how to maximize the use of the device.
A Patient’s Perspective
Larry, a 66-year-old man, was the seventh patient to receive a commercially available Argus II retinal prosthesis in the United States. He started to lose vision in his early 30s, when he was diagnosed with retinitis pigmentosa by Dr. Robert Machemer at the Duke Eye Center in Durham, N.C. At that time, Larry was informed that no medical treatment would prevent his vision loss, which slowly progressed toward profound blindness over the years.
For the past 10 years or so, Larry could not identify if ambient lights were on or off, although clinical assessment with a specialized photoflash test confirmed residual bare light perception vision. Larry was well adapted to his vision loss, but he hoped for more.
Table 4. Argus II Surgical Technique |
| The initial surgery to implant the Argus II may take 3-5 hours (4 hours on average). Surgical steps include: • Crystalline lens removal, if applicable. • Peritomy and muscle isolation. • Placement of extra-ocular portion of device. • Anchoring of suture tabs. • Securing scleral band. • Vitrectomy. • Creation of sclerotomy to insert array. • Tacking of array over macula. • Application of patch graft and closure. |
Following successful implantation, Larry’s journey with the Argus II has been marked with excitement. Three weeks postoperatively, following programming of his VPU, his device was turned on, resulting in visualization of phosphenes (perception of seeing light flashes in response to controlled electrical stimulation of the retina) for the first time in years. (A video of his experience is available on YouTube at https://www.youtube.com/watch?v=CiyGOUHD2nI).
His wife describes what happened one day later: “As we were driving home, Larry turned the device on. He was able to distinguish where the streetlights were, a lit billboard and headlights as they came our way. It was truly amazing!”
One of the most gratifying early experiences for Larry was with lamps in his house. “Before the Argus II, I could not tell if a lamp in the house was on or off without burning my hands,” he said. “Now with the Argus II, I can go around and turn lamps off that are on. That is pretty functional for me!”
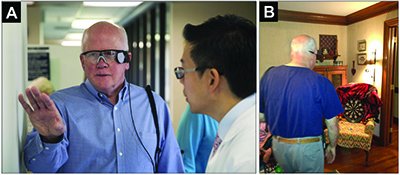
Several weeks into his device, he tried joining his granddaughter in playing Velcro darts (Figure 3). As his wife describes, “I told him he should put on his glasses and see if he could tell where the dart board was. He put on his glasses. Shocker! He found the dart board and started playing. He rarely missed. That’s about as much fun as he has had in a long time playing a game. He wanted to try it again tomorrow and the next day and the next day! To be truthful, I was kind of surprised that he could see it as well as he did. But the darts didn’t lie. He did well!”
 |
With ongoing low vision rehabilitation, Larry has increasingly learned how to use his device to maximize his experiences with it. He is able to identify the location of doorways and windows, he can reach out to find and touch his wife’s and grand children’s faces. He should certainly be able to see the flashes of fireworks. His exploration of a new world with visual stimulation has just begun.
A Future for Artificial Vision
The approval of the Argus II marks an exciting era of enthusiasm and hope for physicians and patients in the treatment of vision previously considered permanently lost.
Although it provides far from natural vision and is currently only approved for patients with profound vision loss from retinitis pigmentosa, the Argus II and other retinal implants and their early successes encourage development of future technologies to provide improved safety and durability, image resolution and applications in other diseases. RS
Disclosures: Dr. Humayun is a consultant, equity owner and holds patents with Second Sight Medical Products, from whom he also receives lecture fees and grant support. Dr. Hahn is a consultant to Second Sight.
References
1. Foerster O. No Beitrage zur Pathophysiologie der Sehbahn und der SehsphareTitle. J Psychol Neurol. 1929;39:463-485.2. Tassicker GE, inventor. Retinal stimulator. US patent 2760483. 1956:2,760,483.
3. Brindley GS, Lewin WS. The sensations produced by electrical stimulation of the visual cortex. J Physiol. 1968;196:479-493.
4. Torab K, Davis TS, Warren DJ, House PA, Normann RA, Greger B. Multiple factors may influence the performance of a visual prosthesis based on intracortical microstimulation: nonhuman primate behavioural experimentation. J Neural Eng. 2011;8:35001.
5. Bi A, Cui J, Ma Y-P, et al. Ectopic expression of a microbial-type rhodopsin restores visual responses in mice with photoreceptor degeneration. Neuron. 2006;50:23-33.
6. Peterman MC, Mehenti NZ, Bilbao K V, et al. The Artificial Synapse Chip: a flexible retinal interface based on directed retinal cell growth and neurotransmitter stimulation. Artif Organs. 2003;27:975-985.
7. Chekhchoukh A, Goumidi M, Vuillerme N, Payan Y, Glade N. Electrotactile vision substitution for 3D trajectory following. Conf Proc Annu Int Conf IEEE Eng Med Biol Soc IEEE Eng Med Biol Soc Annu Conf. 2013;2013:6413-6416.
8. Humayun MS, Dorn JD, da Cruz L, et al. Interim results from the international trial of Second Sight’s visual prosthesis. Ophthalmology. 2012;119:779-788.
9. Chuang AT, Margo CE, Greenberg PB. Retinal implants: a systematic review. Br J Ophthalmol. 2014;98:852-856.
10. Stingl K, Bartz-Schmidt KU, Besch D, et al. Artificial vision with wirelessly powered subretinal electronic implant alpha-IMS. Proc Biol Sci. 2013;280:20130077.
11. Rizzo JF 3rd. Update on retinal prosthetic research: the Boston Retinal Implant Project. J Neuroophthalmol. 2011;31:160-168.
12. Roessler G, Laube T, Brockmann C, et al. Implantation and explantation of a wireless epiretinal retina implant device: observations during the EPIRET3 prospective clinical trial. Invest Ophthalmol Vis Sci. 2009;50:3003-3008.
13. Menzel-Severing J, Laube T, Brockmann C, et al. Implantation and explantation of an active epiretinal visual prosthesis: 2-year follow-up data from the EPIRET3 prospective clinical trial. Eye (Lond). 2012;26:501-509.
14. Matthaei M, Zeitz O, Keserü M, et al. Progress in the development of vision prostheses. Ophthalmologica. 2011;225:187-192.
15. Da Cruz L, Coley BF, Dorn J, et al. The Argus II epiretinal prosthesis system allows letter and word reading and long-term function in patients with profound vision loss. Br J Ophthalmol. 2013;97:632-636.
16. Rizzo S, Belting C, Cinelli L, et al. The Argus II Retinal Prosthesis: 12-month outcomes from a single-study center. Am J Ophthalmol. 2014;157:1282-1290.



