 |
 |
The Diabetic Retinopathy Clinical Research Network Protocol S trial demonstrated that ranibizumab (Lucentis, Roche/Genentech) monotherapy was non-inferior to panretinal photocoagulation at two years in terms of visual acuity outcomes.1 More recently in 2017, the CLARITY trial in the United Kingdom showed us for the first time that aflibercept (Eylea, Regeneron) monotherapy delivered superior VA outcomes at one year compared to PRP.2
In addition, PRP has several potential side effects, including reduced peripheral and night vision, worsening of diabetic macular edema and decreased contrast sensitivity.3–5 Because of these findings, the use of anti-VEGF monotherapy for these patients has gained interest.
LTFU trends for anti-VEGF vs. PRP
Data from clinical trials often do not translate into real-world outcomes, especially in a patient population for whom adherence to treatment recommendations can be challenging. Our institution recently looked at rates of lost to follow-up (LTFU) in 2,302 patients with proliferative diabetic retinopathy after receiving either anti-VEGF vs. PRP.6
We found that about a quarter of patients with PDR were LTFU after at least one treatment session for a year or more. We identified some risk factors for LTFU in this study (see “Why PDR Patients May Not Come Back,” November 2018 Retina Specialist), but the study also raised two important questions:
• What happened to these patients when they returned to our office?
• Was there a difference in outcome if they had received only anti-VEGF vs only PRP?
Therefore, we conducted a study that sought to evaluate the outcomes between eyes that received only anti-VEGF therapy vs. PRP and were LTFU for more than six months after their procedure.
Characteristics of LTFU population
Seventy-six eyes were eligible for inclusion in our study. Thirty eyes received anti-VEGF and 46 received PRP prior to LTFU. Of those that received anti-VEGF, 11 received bevacizumab (Avastin, Roche/ Genentech), five ranibizumab and 14 aflibercept. The anti-VEGF group received a mean of five injections prior to LTFU, and the PRP group received a mean of two PRP sessions.
The mean duration of LTFU was 371 days for the anti-VEGF group and 465 days for the PRP group. After returning from LTFU and resuming therapy, the anti-VEGF group received an average of four additional injections and one PRP session between the return visit and the final visit. The PRP group received an average of one injection and one additional PRP session between the return and final visit.
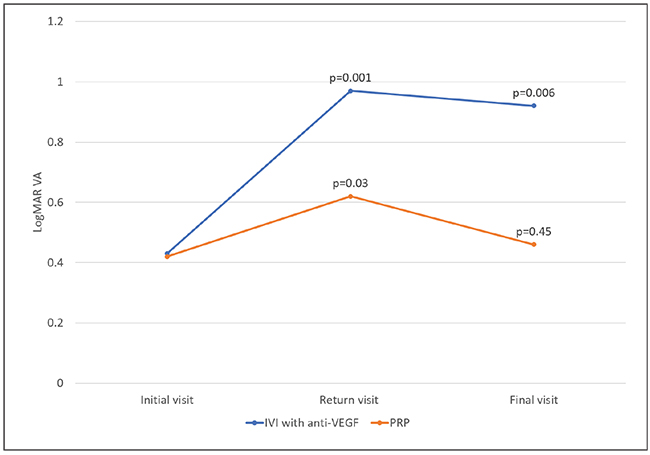 |
| Figure. At the visit before patients were lost to follow-up (LTFU), visual acuity in both the anti-VEGF and panretinal photocoagulation groups were about the same. On the return visit after LTFU, VA worsened significantly in both groups, but eventually returned to baseline in the PRP group. (Used with permission Obeid A, Su D, Patel SN, et al. Ophthalmology. 2018 August 2.[Epub ahead of print]). |
 |
Anatomic, visual outcomes
At the visit prior to LTFU, visual acuity in both groups were very similar (20/53; Figure). After being LTFU, VA worsened significantly in both groups (20/187 for anti-VEGF and 20/83 for PRP). However, VA in the PRP group eventually returned to baseline (20/58) after additional therapy, while VA in the anti-VEGF group remained significantly worse compared to baseline (20/166).
Due to the retrospective nature of the study and current practice patterns, this is in the context of a higher proportion of eyes with DME in the anti-VEGF group at all three time points (86 percent in the anti-VEGF group vs. 16 percent in the PRP group at baseline).
In terms of other anatomic outcomes, the presence of vitreous hemorrhage was similar between the two groups at all three study time points. Thirteen percent of eyes in the anti-VEGF group required vitrectomy for vitreous hemorrhage vs. 9 percent in the PRP group.
Most striking finding
Perhaps the most striking outcome of this study is the difference in the incidence of tractional retinal detachments (TRD) and neovascular glaucoma (NVG) between the two groups. At the visit prior to LTFU, there was one TRD in the anti-VEGF group and none in the PRP group. At the return visit, 17 percent of eyes in the anti-VEGF group developed a TRD (which increased to more than 30 percent of eyes by the final visit). In comparison, no eyes in the PRP group had a TRD at the return visit and only one eye (2 percent) had a TRD by the final visit.
 |
This translates to around 20 percent of eyes requiring vitrectomy for TRD repair in the group treated with anti-VEGF vs. none in the group treated with PRP. There were also four cases of iris neovascularization with two developing NVG in the anti-VEGF group by the final visit vs. none in the PRP group.
 |
Bottom line
While anti-VEGF injections may deliver superior visual outcomes when follow-up is consistent, this is not always feasible in the real world where patients in this population are at particularly high risk of being LTFU for prolonged time periods. This is especially concerning given that the effect of anti-VEGF may only last one month after intravitreal delivery.7
On the other hand, PRP is known to have a long-term effect. More than 80 percent of eyes in the Early Treatment Diabetic Retinopathy Study retained 20/40 or better vision after 16.5 years of follow up.8
When determining the choice of treatment, clinicians must weigh the benefit of anti-VEGF monotherapy against the risk of potentially vision-threatening outcomes if the patient fails to follow up.
In the future, longer acting anti-VEGF therapies or delivery systems may eventually sway the treatment paradigm toward anti-VEGF monotherapy, but for now therapy with PRP may be more protective long term in case patients are LTFU. RS
REFERENCES
1. Writing Committee for the Diabetic Retinopathy Clinical Research Network, Gross JG, Glassman AR, et al. Panretinal photocoagulation vs intravitreous ranibizumab for proliferative diabetic retinopathy: a randomized clinical trial. JAMA 2015;314:2137–2146.
2. Sivaprasad S, Prevost AT, Vasconcelos JC, et al. Clinical efficacy of intravitreal aflibercept versus panretinal photocoagulation for best corrected visual acuity in patients with proliferative diabetic retinopathy at 52 weeks (CLARITY): a multicentre, single-blinded, randomised, controlled, phase 2b, non-inferiority trial. Lancet 2017;389:2193–2203.
3. No authors listed. Photocoagulation treatment of proliferative diabetic retinopathy. Clinical application of Diabetic Retinopathy Study (DRS) findings, DRS Report Number 8. The Diabetic Retinopathy Study Research Group. Ophthalmology 1981;88:583–600.
4. Diabetic Retinopathy Clinical Research Network, Brucker AJ, Qin H, Antoszyk AN, et al. Observational study of the development of diabetic macular edema following panretinal (scatter) photocoagulation given in 1 or 4 sittings. Arch Ophthalmol 2009;127:132–140.
5. Preti RC, Ramirez LMV, Monteiro MLR, et al. Contrast sensitivity evaluation in high risk proliferative diabetic retinopathy treated with panretinal photocoagulation associated or not with intravitreal bevacizumab injections: a randomised clinical trial. Br J Ophthalmol 2013;97:885–889.
6. Obeid A, Gao X, Ali FS, et al. Loss to follow-up among patients with neovascular age-related macular degeneration who received intravitreal anti-vascular endothelial growth factor injections. JAMA Ophthalmol 2018;136:1251–1259.
7. Krohne TU, Liu Z, Holz FG, Meyer CH. Intraocular pharmacokinetics of ranibizumab following a single intravitreal injection in humans. Am J Ophthalmol 2012;154:682-686.e2.
8. Aiello LP, Avery RL, Arrigg PG, et al. Vascular endothelial growth factor in ocular fluid of patients with diabetic retinopathy and other retinal disorders. N Engl J Med 1994;331:1480–1487.



