| ABOUT THE AUTHORS | |
 | Dr. Efrem Mandelcorn is an attending vitreoretinal surgeon at the Toronto Western Hospital of the University of Toronto University Health Network. He was fellowship-trained at the University of Toronto and the Bascom Palmer Eye Institute, University of Miami. Dr. Mark Mandelcorn is a retina specialist practicing surgical and medical retina at Toronto Western Hospital of the University of Toronto, where he has been involved in clinical teaching, research and administration. Dr. Manusow is a vitreoretinal surgery fellow at the University of Toronto, where he also completed his ophthalmology residency and served as chief resident. DISCLOSURES: The authors have no relevant disclosures. |
 | |
 | |
Indications and Patient Selection
Classically, PR was indicated in the following types of retinal detachments (RD): a single break no larger than one clock hour located in the superior eight clock hours of the ocular fundus; or a group of small breaks within one clock hour without grade C or D proliferative vitreoretinopathy, glaucoma, retinal breaks in the inferior four clock hours or in a patient unable to comply with head positioning.2,3Many retinal surgeons have attempted to expand the use of PR beyond these classic 1986 indications to treat recurrent detachments following scleral buckle (SB) or pars plana vitrectomy (PPV) to include inferior retinal breaks, giant retinal tears and dialyses.4-9
Another not yet reported indication is to use pneumatic retinopexy to keep the macula attached in a macula-on RD so that vitrectomy or scleral buckling surgery can be delayed to a more appropriate time when the proper surgical team and resources are in place. By injecting the gas bubble and positioning the patient face down, the macula will remain attached until a more definitive procedure can take place.
Anecdotally, we have done this procedure with great success when, for various reasons, we cannot get a patient into the operating room as fast as we would like or if the patient needs to delay the procedure.
Our Current Technique
Before performing a PR, the surgeon must note anterior segment findings such as lens status and chamber depth. A detailed fundus drawing noting the number, size and location of all retinal breaks, subretinal fluid accumulation and the presence of retinal breaks or lattice degeneration in the areas of attached retina is essential.10,11These are the key steps and considerations involved in our approach to performing pneumatic retinopexy:12
• Laser retinopexy. Before the procedure, we barricade with laser any retinal breaks or lesions predisposing to retinal breaks, such as lattice degeneration in the flat retina.
• Anesthesia and asepsis. We begin with anesthetic eye drops followed with either xylocaine gel or subconjunctival lidocaine injection. Then we insert an eyelid speculum and instill a drop of povidone 5% into the conjunctival sac, especially over the area of the intended injection site.
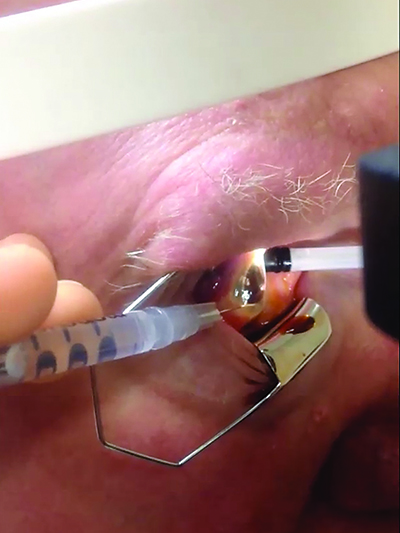
or 27-gauge needle horizontally at the inferotemporal limbus, creating a
beveled entry that is self-sealing. Applying pressure on the nasal-scleral side
of the entry site facilitates drainage of fluid from the anterior chamber. Online
video available at http://goo.gl/6BfBoc.
• Intraocular gas injection. The needle should enter at the highest point of the eye. Some prefer injecting the gas at the 12 o’clock position while others inject in the superonasal or superotemporal quadrant away from the most bullous area of detachment. We inject 4 mm from the limbus in phakic patients and 3.5 mm from the limbus in pseudophakic patients.
We lean the patient back in the examining chair and ask him to look down. We make the injection using a slow continuous movement on the syringe plunger so that a gas bubble develops at the tip of the needle in the vitreous cavity and the needle remains in the bubble as it expands with more gas. The needle and syringe are removed in a quick movement to allow the external puncture opening to self-seal. Some surgeons use a sterile cotton-tipped applicator to immediately massage the puncture site and avoid gas leakage.
• Choice and volume of injected gas. We use SF6 or C3F8 gas, injecting 0.6 ml of the former or 0.3 ml of the latter. The bubble will slowly expand over days to a volume of 1.2 ml allowing for slow equilibration of intraocular pressure.13
• Control of intraocular pressure after gas injection. If the optic nerve is not perfused immediately following the gas injection, we make another paracentesis with the 27- or 30-gauge needle through the original paracentesis site. Perfusion of the nerve must be verified before the patient leaves.
• Assessing the size and adequacy of the gas bubble. Using the indirect ophthalmolscope, we assess the size and number of the gas bubble(s). The procedure is finished.
Care After the Injection
Care of the patient following the injection involves the following steps:• Post-injection positioning. The goal of positioning is to allow the gas bubble to tamponade the retinal break. Some retinal surgeons carry out a “steamroller” maneuver to try to keep subretinal fluid from tracking into the macula. The patient is instructed to begin positioning immediately.
• Laser retinopexy 24 to 48 hours later. If the retina around the retinal breaks is now attached, it is possible to administer laser photocoagulation around the retinal breaks within two days after the injection. The laser indirect ophthalmoscope, in our hands, is the best method of doing this. The patient continues positioning for another several days to ensure that a firm chorioretinal adhesion forms in the laser-treated area.14
Troubleshooting
Even with the best-planned procedures, problems and complications can occur. Here is how we avoid the most common postoperative issues and manage them if they do occur.• “Fish eggs.” Multiple small bubbles called “fish eggs”1 are a nuisance because they can potentially gain access to the subretinal space through a large break, or make visualization extremely difficult. A few small bubbles are sometimes unavoidable and usually will coalesce over one to two days. If not, the eye can be gently tapped or flicked to encourage this.
To avoid fish eggs, we recommend you ensure the needle is at the highest point of the globe when injecting. We generally push the needle one-third of the way in, and then withdraw it until the tip is just in the vitreous cavity. Making the injection with steady pressure and the needle stationary will help keep the tip within the expanding bubble and prevent fish eggs.
If you encounter fish eggs, simply position the patient in a way as to move them out of view by adjusting the angle of the chair or putting the patient on his side during the laser retinopexy in order to achieve better visualization. Fish eggs can still tamponade a retinal break, and we have seen many retinas successfully reattached despite multiple small bubbles.
• Sub-retinal fluid despite a flat break. Not all retinal pigment epithelium is created equal. A not-uncommon scenario we see is that the patient returns 48 hours after gas injection with a flat retinal break but persistent subretinal fluid elsewhere. In such a case, it is important to thoroughly reexamine the eye to ensure no open breaks exist. If there are none, we laser barricade the flat break and observe patiently.
Some patients have a weak RPE pump that prolongs the time it can take for the retina to flatten. We have waited days to weeks for some retinas to fully flatten. The goal of PR is to flatten the area of retinal break so that it can be barricaded. It is helpful to be patient with persistent subretinal fluid as long as no open retinal breaks are present.
• A perfect candidate but failed procedure. Occasionally, we perform PR on the perfect candidate with a phakic superior RD and one small break, but the retina does not flatten around the break. If examination of the fundus does not reveal a missed open break, the problem may be poor patient positioning.
Better physician communication can remedy this. We often write down specific positioning instructions or place an arrow on the patient’s forehead to clearly indicate the correct position to maintain. Sometimes, we also have the patient position in the office before he leaves to ensure he has it right. This allows us to ensure that the bubble is tamponading the break with direct visualization.
It is sometimes helpful to have the patient position on one side or the other and not face down because side positioning often covers at least four or five clock hours of temporal or nasal retina.
• Difficulty with laser retinopexy. Skill with indirect laser retinopexy is essential to performing PR well. This is by far the most technically challenging part of the procedure and requires practice. To avoid difficulty with visualization secondary to the refractive change at the edge of the bubble, position the patient to either laser through the center of the bubble or turn the patient’s head while the he is supine so that the bubble moves completely out of view.
Once the retina flattens, it can sometimes be difficult to identify small holes that were easier to see in the detached retina. Careful fundus drawing, especially noting adjacent landmarks such as blood vessels and pigmentary changes that could aid in finding the break later, also help.
Marking the location of the break in an area of adjacent attached retina prior to injection of the gas bubble is also helpful. We do this by placing a laser burn either in the adjacent flat retina or the pars plana. Cryotherapy prior to gas injection can also help to eliminate the need to find and treat the break again after gas injection. However, we prefer to perform laser because it less inflammatory.
Finally, if you cannot identify the break definitively, you can apply diffuse peripheral laser in the suspected area.
• Inferior retinal breaks. We have performed many PR procedures for patients with inferior retinal breaks for various reasons, including patient preference, patient age and lens status. Although it’s not as successful as it has been in patients with more classic indications, we have had good results.
In our experience, positioning the patient directly on his side can usually achieve tamponade of inferior retinal breaks, which allows the gas bubble to cover the inferotemporal or inferonasal retina.
In cases of 6 o’clock breaks, a second gas bubble injection 24-48 hours after the first provides tamponade to the additional inferior clock hours to 6 o’clock when the patient is positioned on his side.
Serial pneumatic retinopexy, or a “double bubble,” can be performed in the same way as a single procedure, 24 to 48 hours later. This involves performing an AC tap to make room for the second bubble. This second bubble is then injected into the first fully expanded gas bubble to achieve a single larger bubble.
Outcomes and Results
| Take-home Point Pneumatic retinopexy is an in-office procedure that, in our hands, works well to reattach a majority of retinal detachments. It is minimally invasive with few complications, with little downside to employing it as an initial procedure. The key to success is experience, patient selection, patience and communication. |
In reality, success with PR requires much more manipulation of gas bubble positioning and sometimes supplementation with sequential gas bubble injections as well as more than one laser treatment. Other studies have reported retinal reattachment rates of 60 to 91 percent.2,8,11,15-18
Inexpensive Yet Unpopular
Economic analyses have shown that PR is more than 50 percent less expensive than scleral buckling or pars plana vitrectomy, and has the most utility when dollars/quality-of-life-year saved is studied.19-20 Despite this, recent surveys found only 25 percent of retina specialists would use PR for a retinal detachment with a single superior break, which is a decline from previous data.21Overall, only 15 percent of retinal detachments are treated with PR in the United States, and studies have estimated that Medicare would save $6 million to $30 million if this rate increased to just 20 to 35 percent.19,22
Conclusion
We have found little downside to employing pneumatic retinopexy as an initial procedure and we would choose to undergo it before vitrectomy or scleral buckle were our own retinas to become detached. We recommend retina specialists make it a part of their practice. RSReferences
1. Feng H, Adelman RA. Cataract formation following vitreoretinal procedures. Clin Ophthalmol. 2014;8:1957-1965.
2. Tornambe PE, Hilton GF. Pneumatic retinopexy. A multicenter randomized controlled clinical trial comparing pneumatic retinopexy with scleral buckling. The Retinal Detachment Study Group. Ophthalmology. 1989;96:772-784.
3. Hilton GF, Das T, Majji AB, Jalali S. Pneumatic retinopexy: principles and practice. Indian J Ophthalmol. 1996;44:131-143.
4. Hilton GF, Grizzard WS. Pneumatic retinopexy. A two-step outpatient operation without conjunctival incision. Ophthalmology. 1986;93:626-641.
5. Friberg TR, Eller AW. Laser pneumatic retinopexy for repair of recurrent retinal detachment after failed scleral buckle--ten years experience. Ophthalmic Surg Lasers. 2001;32:13-18.
6. Modi YS, Townsend J, Epstein AE, et al. Pneumatic retinopexy for retinal detachment occurring after prior scleral buckle or pars plana vitrectomy. Ophthalmic Surg Lasers Imaging Retina. 2014;45:409-413.
7. Chang TS, Pelzek CD, Nguyen RL, et al. Inverted pneumatic retinopexy: A method of treating retinal detachments associated with inferior retinal breaks. Ophthalmology. 2003;110:589-594.
8. Tornambe PE, Hilton GF, Kelly NF, et al. Expanded indications for pneumatic retinopexy. Ophthalmology. 1988;95:597-600.
9. Melgen SE, Michels M. Pneumatic retinopexy for the treatment of giant retinal dialyses. Am J Ophthalmol. 1994;118:762-765.
10. Tornambe PE. Pneumatic retinopexy: The evolution of case selection and surgical technique. A twelve-year study of 302 eyes. Trans Am Ophthalmol Soc. 1997;95: 551-578.
11.Rootman DB, Luu S, M Conti S, et al. Predictors of treatment failure for pneumatic retinopexy. Can J Ophthalmol. 2013;48:549-552.
12. Mandelcorn E, Mandelcorn M, Manusow JS. Update on pneumatic retinopexy. Curr Opinion Ophthalmol. 2015;26:194-199.
13. Lincoff H, Kreissig I, Brodie S, Wilcox L. Expanding gas bubbles for the repair of tears in the posterior pole. Graefes Arch Clin Exp Ophthalmol. 1982;219:193-197.
14. Poliner LS, Tornambe PE. Failed retinal detachment repair after intravitreal air injection. Arch Ophthalmol. 1989;107:487-488.
15. Hilton GF, Kelly NE, Salzano TC, Tornambe PE, Wells JW, Wendel RT. Pneumatic retinopexy. A collaborative report of the first 100 cases. Ophthalmology. 1987;94: 307-314.
16. Tornambe PE, Hilton GF, Brinton DA, et al. Pneumatic retinopexy. A two-year follow-up study of the multicenter clinical trial comparing pneumatic retinopexy with scleral buckling. Ophthalmology. 1991;98:1115-1123.
17. Fabian ID, Kinori M, Efrati M, et al. Pneumatic retinopexy for the repair of primary rhegmatogenous retinal detachment: A 10-year retrospective analysis. JAMA Ophthalmol. 2013;131:166-171.
18. Gilca M, Duval R, Goodyear E, et al. Factors associated with outcomes of pneumatic retinopexy for rhegmatogenous retinal detachments: A retrospective review of 422 cases. Retina. 2014;34:693-699.
19. Goldman DR, Shah CP, Heier JS. Expanded criteria for pneumatic retinopexy and potential cost savings. Ophthalmology. 2014;121:318-326.
20. Chang JS, Smiddy WE. Cost-effectiveness of retinal detachment repair. Ophthalmology 2014;121:946-951.
21. Williams PD, Hariprasad SM. Evolving trends in primary retinal detachment repair: Microincisional vitrectomy and the role of OCT. Ophthalmic Surg Lasers Imaging Retina. 2014;45:268-272.
22. Hwang JC. Regional practice patterns for retinal detachment repair in the United States. Am J Ophthalmol. 2012;153:1125-1128.
2. Tornambe PE, Hilton GF. Pneumatic retinopexy. A multicenter randomized controlled clinical trial comparing pneumatic retinopexy with scleral buckling. The Retinal Detachment Study Group. Ophthalmology. 1989;96:772-784.
3. Hilton GF, Das T, Majji AB, Jalali S. Pneumatic retinopexy: principles and practice. Indian J Ophthalmol. 1996;44:131-143.
4. Hilton GF, Grizzard WS. Pneumatic retinopexy. A two-step outpatient operation without conjunctival incision. Ophthalmology. 1986;93:626-641.
5. Friberg TR, Eller AW. Laser pneumatic retinopexy for repair of recurrent retinal detachment after failed scleral buckle--ten years experience. Ophthalmic Surg Lasers. 2001;32:13-18.
6. Modi YS, Townsend J, Epstein AE, et al. Pneumatic retinopexy for retinal detachment occurring after prior scleral buckle or pars plana vitrectomy. Ophthalmic Surg Lasers Imaging Retina. 2014;45:409-413.
7. Chang TS, Pelzek CD, Nguyen RL, et al. Inverted pneumatic retinopexy: A method of treating retinal detachments associated with inferior retinal breaks. Ophthalmology. 2003;110:589-594.
8. Tornambe PE, Hilton GF, Kelly NF, et al. Expanded indications for pneumatic retinopexy. Ophthalmology. 1988;95:597-600.
9. Melgen SE, Michels M. Pneumatic retinopexy for the treatment of giant retinal dialyses. Am J Ophthalmol. 1994;118:762-765.
10. Tornambe PE. Pneumatic retinopexy: The evolution of case selection and surgical technique. A twelve-year study of 302 eyes. Trans Am Ophthalmol Soc. 1997;95: 551-578.
11.Rootman DB, Luu S, M Conti S, et al. Predictors of treatment failure for pneumatic retinopexy. Can J Ophthalmol. 2013;48:549-552.
12. Mandelcorn E, Mandelcorn M, Manusow JS. Update on pneumatic retinopexy. Curr Opinion Ophthalmol. 2015;26:194-199.
13. Lincoff H, Kreissig I, Brodie S, Wilcox L. Expanding gas bubbles for the repair of tears in the posterior pole. Graefes Arch Clin Exp Ophthalmol. 1982;219:193-197.
14. Poliner LS, Tornambe PE. Failed retinal detachment repair after intravitreal air injection. Arch Ophthalmol. 1989;107:487-488.
15. Hilton GF, Kelly NE, Salzano TC, Tornambe PE, Wells JW, Wendel RT. Pneumatic retinopexy. A collaborative report of the first 100 cases. Ophthalmology. 1987;94: 307-314.
16. Tornambe PE, Hilton GF, Brinton DA, et al. Pneumatic retinopexy. A two-year follow-up study of the multicenter clinical trial comparing pneumatic retinopexy with scleral buckling. Ophthalmology. 1991;98:1115-1123.
17. Fabian ID, Kinori M, Efrati M, et al. Pneumatic retinopexy for the repair of primary rhegmatogenous retinal detachment: A 10-year retrospective analysis. JAMA Ophthalmol. 2013;131:166-171.
18. Gilca M, Duval R, Goodyear E, et al. Factors associated with outcomes of pneumatic retinopexy for rhegmatogenous retinal detachments: A retrospective review of 422 cases. Retina. 2014;34:693-699.
19. Goldman DR, Shah CP, Heier JS. Expanded criteria for pneumatic retinopexy and potential cost savings. Ophthalmology. 2014;121:318-326.
20. Chang JS, Smiddy WE. Cost-effectiveness of retinal detachment repair. Ophthalmology 2014;121:946-951.
21. Williams PD, Hariprasad SM. Evolving trends in primary retinal detachment repair: Microincisional vitrectomy and the role of OCT. Ophthalmic Surg Lasers Imaging Retina. 2014;45:268-272.
22. Hwang JC. Regional practice patterns for retinal detachment repair in the United States. Am J Ophthalmol. 2012;153:1125-1128.



