• pachychoroid pigment epitheliopathy (PPE);
• central serous chorioretinopathy (CSCR); pachychoroid neovasculopathy (PNV); and
• polypoidal choroidal vasculopathy (PCV).
Here, we review the four groups of the pachyroid spectrum.
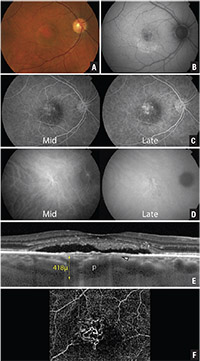 |
| Figure 1. Multimodal imaging of an asymptomatic 44-year-old female patient with pachychoroid pigment epitheliopathy. Fundus photographs (A) of both eyes show bilateral retinal pigment epithelial and pigmentary changes. Fundus autofluorescence (B) demonstrates patches of hyperautofluorescence and hypoautofluorescence at both macular regions. Optical coherence tomography (C) of both eyes reveals a thick choroid bilaterally and a small left parafoveal serous pigment epithelial detachment. |
Before we discuss each group, it is important to understand the anatomical features of the normal choroid and how the pachychoroid disease spectrum alters them. The choroid is a vascularized and pigmented tissue, which is in continuation with the ciliary body and iris. It surrounds the optic nerve head posteriorly and extends anteriorly to the ora serrata. The choroid has a mean thickness of 0.15 mm anteriorly and 0.22 mm posteriorly.3
From the outside to inside, the choroid consists of five layers:
• suprachoroid lamina;
• Haller’s layer (large choroidal
veins);
• Sattler’s layer (medium-sized
choroidal veins);
• choriocapillaris (fenestrated
vessels that supply the outer
retina); and
• Bruch’s membrane (a thin membrane adjacent to the retinal pigment epithelium). A structurally and functionally intact choroid is important for normal retinal function, as the choroidal blood supply nourishes the external third of the retina.
Disease Characteristics
Choroidal thickness varies with age, ethnicity and axial length, and normal mean subfoveal choroidal thickness varies between 191 and 354 μm. A thick choroid, the basis of the “pachychoroid” term, can be defined as a choroidal thickness of >390 μm.4 Although clinical manifestations of pachychoroid spectrum disorders vary considerably, sweptsource optical coherence tomography has shown that they share similar morphological findings in the choroid, namely increased thickness and dilated outer choroidal vessels.5 With disease chronicity, focal choriocapillaris atrophy with inward displacement of deep choroidal vessels ensues.
The advent of enhanced depth imaging (EDI) OCT, swept-source OCT and fundus autofluorescence has led to greater precision in the diagnosis of pachychoroid-driven diseases, which previously were easy to misdiagnose or overlook.
 |
PPE is thought to represent a form fruste or precursor of CSCR, as it has features of retinal pigment epithelium disturbances similar to CSCR but without clinical or imaging evidence of acute or chronic subretinal fluid.1 It is typically a “silent” disease because patients are frequently asymptomatic. Patients with PPE possess a thickened choroid, appearing clinically as a reddish-orange fundus, with reduced fundus tessellation and a variety of overlying RPE abnormalities including small pigment epithelial detachments.
In the setting of unilateral CSCR, reports have documented PPE findings such as pigmentary changes, asymptomatic PEDs, choroidal hyperpermeability and increased choroidal thickness in the fellow eye, even without serous neurosensory detachment.6
Idiopathic serous PEDs have also been implicated as a subtle variant of CSCR.7 Alphonso Giovannini, MD, and colleagues analyzed indocyanine green angiography videos of 25 patients with idiopathic serous PEDs and found that 83 percent of eyes had evidence of choroidal hyperpermeability.8 Furthermore, 33 percent of eyes had an irregularly dilated choroidal vein within 1 disc diameter of the PEDs. This finding suggests that a thickened choroid drives the RPE changes seen in PPE.
In the clinical setting, PPE can be identified based on clinical findings and noninvasive tests such as OCT and FAF, as demonstrated by our index case (Figure 1). This case involves an asymptomatic 44-yearold woman, who was referred with pigmentary changes in both macular regions. Her investigative findings were consistent with PPE.
A greater awareness of this entity may encourage clinicians to perform these investigations and make the diagnosis earlier, which may ultimately prevent unnecessary diagnostic tests and interventions. Patients with PPE are frequently misdiagnosed as having inactive CSCR, early age-related macular degeneration, macular dystrophies or inflammatory chorioretinopathies such as punctate inner choroidopathy.
Central Serous Chorioretinopathy
CSCR is a retinal disorder that occurs in otherwise healthy young or middle-aged patients. The disease is characterized by serous detachment of the neurosensory retina, which is often associated with one or more serous PEDs. In keeping with its inclusion in the pachychoroid disease spectrum, patients with CSCR have a thick choroid, increased choroidal vascular area and increased choroidal vascular permeability.9,10
Pigment epithelial detachments may occur in areas of choroidal hyperpermeability, increasing the risk of local RPE disruption, eventually resulting in leakage of serous fluid into the subretinal space. It is not surprising that CSCR risk factors, such as type A personality traits, corticosteroid exposure and pregnancy, are also risk factors for PPE development.1
Pachychoroid Neovasculopathy
The PPE and CSCR spectrum of pachychoroid diseases may progress to PNV, a form of Type 1 (sub-RPE) neovascularization occurring over areas of increased choroidal thickness and dilated choroidal vessels. Although the exact mechanism has not been elucidated, authors have theorized it is similar to the process in AMD, in which a break in Bruch’s membrane, due to chronic RPE changes and longstanding PEDs, allows the ingrowth of abnormal blood vessels.
Claudine Pang, MD, and colleagues reported a case series of three patients with PNV.2 Because these patients don’t have typical AMD or degenerative changes, they are often diagnosed with choroidal neovascularization from an unknown etiology. In this series, OCT imaging revealed not only choroidal thickening, but also choroidal vascular dilation directly below the neovascular tissue and obliteration of the choriocapillaris and Sattler’s layers. Of note, all three patients were younger than the typical age of presentation for AMD-related CNV.
Kunal Dansingani, MD, and colleagues in another study used OCT-A and dye angiography to examine 22 eyes with pachychoroid features and shallow irregular PEDs.11 They identified PCV in four eyes with dye angiography, and only 29 percent of the remaining eyes demonstrated specific features of neovascularization. In contrast, they also found Type 1 neovascularization on OCT-A in 21 of the 22 study eyes.
Hence, the finding of irregular, shallow PEDs and a thick choroid on OCT has greater diagnostic value for Type 1 neovascularization than previously thought. More importantly, dye angiography may underestimate the presence of neovascularization compared to OCT-A in this disease. Figure 2 (page 46) demonstrates the multimodal imaging results of one of the patients Dr. Dansingani and colleagues included in their study.
 |
| Figure 2. Multimodal imaging of the right eye of a 63-year-old female with pachychoroid neovasculopathy from Kunal Dansingani, MD, and colleagues.11 Color photograph (A) and fundus autofluorescence of the right eye show non-specific retinal pigment epithelium changes with a dependent morphology. Mid-and late-phase fluorescein angiography (C) reveal an area of poorly defined hyperfluorescence with minimal late leakage. Indocyanine green angiography (D) shows large, dilated choroidal vessels (pachyvessels) with focal choroidal hyperpermeability, but a late-phase plaque is not definitely visible. Optical coherence tomography (E) shows subretinal fluid, subfoveal debris and pachyvessels (p) with overlying choriocapillaris thinning (white arrow) and a shallow, irregular PED. En-face OCT angiography (F) through the pigment epithelial detachment shows a tangled network of type 1 neovascular tissue. (Used with permission. Dansin- gani KK, et al. Am J Ophthalmol. 2015;164:1243-1254 e2.) |
The link between CSCR and PCV has been well documented. The strikingly similar characteristics of the two diseases—namely increased choroidal permeability as seen with ICGA, increased choroidal thickness as demonstrated with EDI-OCT and histopathological findings of thin-walled choroidal vessels in PCV—support the theory that CSCR and PCV may be part of a pachychoroid-driven disease spectrum.2
Studies have suggested that PCV may be a manifestation of long-standing type 1 neovascularization. Samira Khan, MD, and colleagues showed that PCV can develop at the margins of long-standing Type 1 CNV in a variety of macular diseases.12
In another series of 27 eyes, Adrian Fung, MD, and colleagues showed that 36 percent of their patients with chronic CSCR and Type 1 neovascularization went on to develop PCV.13 Reiterating the finding of increased choroidal permeability in PCV, researchers in Japan found that multifocal choroidal hyperfluorescence on ICGA occurs more frequently in patients with PCV than in those with AMD.14
Previous histological and imaging studies found that the neovascular complexes in PCV are situated between the RPE and Bruch’s membrane.15 This further supports the theory that PCV is not a primary choroidopathy, but rather a secondary neovasculopathy and a variant of Type 1 neovascularization. The presence of vessel hyalinization, which is characteristic of PCV, implies the chronicity of the neovascular complexes.16 The chronicity of PCV vessels and the findings of increased choroidal thickness and permeability in PCV suggest links between chronic CSCR, PNV and PCV.
Causative Theories
In pachychoroid spectrum diseases, the spatial distribution of RPE changes, neurosensory retinal detachment and neovascularization seem to correlate with localized choroidal thickening, vessel dilation of areas of Haller’s layer and thinning of the choriocapillaris and Sattler’s layers.5
A study using SS-OCT and OCT-A showed that the location of pathologically dilated Haller’s layer vessels correlated to zones of reduced choriocapillaris flow, which implies that inner-choroidal ischemia seems to be related to the pathogenesis of pachychoroid diseases.17 On a different note, another group hypothesized that impeded choroidal vascular outflow due to a thickened sclera and increased scleral rigidity causes the pachychoroid spectrum diseases in part.18
Evidence of the role genetics plays in the development of pachychoroid-related diseases has been increasing. Mathieu Lehmann, MD, and colleagues in France studied five CSCR patients and their relatives with enhanced depth OCT and found that 50 percent of the eyes from relatives had a thick choroid.4 This observation suggests that the pachychoroid trait could potentially be a dominantly inherited condition, predisposing to CSCR depending on other exogenous or endogenous factors. Interestingly, a Japanese study also reported that the genetic background of patients with choroidal hyperpermeability and Type 1 neovascularization was different from those with AMD.19
Potential Therapeutic Options
Improved detection and recognition of the more severe manifestations of the pachychoroid disease spectrum has important treatment implications. Two treatment options have emerged: photodynamic therapy and anti-VEGF injections.
• PDT. As evident in the EVEREST II study, PDT has established itself as an important therapeutic consideration for PCV.20 Additionally, sufficient evidence suggests that PDT may be a useful treatment option for chronic CSCR.21 Because PNV exists on the spectrum of CSCR to PCV, PDT may be a viable therapeutic option for this disease as well.
• Anti-VEGF therapy. This treatment has to be considered when Type 1 neovascularization is present with pachychoroid related diseases. A group from Spain treated 18 consecutive unilateral PNV patients with anti-VEGF injections (ranibizumab [Lucentis, Roche/Genentech] or aflibercept [Eylea, Regeneron]) and found that choroidal thickness decreased significantly from baseline to month 12 in treated eyes.22 The subfoveal choroidal thickness of treated eyes decreased from a mean of 318 μm at baseline to 267 μm at 12 months. Based on this finding, the authors hypothesized that anti-VEGF injections may reduce choroidal vascular permeability and, thus, decrease PNV activity.
Another group from Japan demonstrated that a treat-and-extend regimen of intravitreal aflibercept is effective in terms of vision improvement and resolution of exudative changes in both PNV and eyes with Type 1 neovascular AMD.23 Interestingly, eyes with PNV required fewer injections, and among eyes with PNV, those with polypoidal lesions required fewer injections than those without polypoidal lesions.
Conclusion
PPE, CSCR and PCV fall within a spectrum of pachychoroid related disease that can result in RPE changes, branching vascular networks, Type 1 choroidal nevascularization (PNV) and PCV. Recognition and identification of the earlier stages of the disease spectrum could potentially avoid mismanagement or over-investigation. For the more advanced stages of the disease spectrum, therapies that target the pachychoroid aspect of the disease, such as PDT and anti-VEGF injections, could potentially help achieve long-term disease stability.
Dr. Mandelcorn is an associate professor of ophthalmology at the University of Toronto. Dr. Yap is a vitreoretinal fellow there.
References
1. Warrow DJ, Hoang QV, Freund KB. Pachychoroid pigment epitheliopathy. Retina. 2013;33:1659-1672.
2. Pang CE, Freund KB. Pachychoroid neovasculopathy. Retina. 2015;35:1-9.
3. Akkaya S. Spectrum of pachychoroid diseases. Int Ophthalmol. 2017 Aug 1 [Epub ahead of print].
4. Lehmann M, Bousquet E, Beydoun T, Behar-Cohen F. Pachychoroid: an inherited condition? Retina. 2015;35:10-16.
5. Dansingani KK, Balaratnasingam C, Naysan J, Freund KB. En face imaging of pachychoroid spectrum disorders with sweptsource optical coherence tomography. Retina. 2016;36:499516.
6. Gupta P, Gupta V, Dogra MR, Singh R, Gupta A. Morphological changes in the retinal pigment epithelium on spectral-domain OCT in the unaffected eyes with idiopathic central serous chorioretinopathy. Int Ophthalmol. 2010;30:175-181.
7. Wang M, Munch IC, Hasler PW, Prunte C, Larsen M. Central serous chorioretinopathy. Acta Ophthalmol. 2008;86:126-145. 8. Giovannini A, Scassellati-Sforzolini B, D’Altobrando E, Mariotti C, Rutili T, Tittarelli R. Choroidal findings in the course of idiopathic serous pigment epithelium detachment detected by indocyanine green videoangiography. Retina. 1997;17:286-293. 9. Imamura Y, Fujiwara T, Margolis R, Spaide RF. Enhanced depth imaging optical coherence tomography of the choroid in central serous chorioretinopathy. Retina. 2009;29(10):1469-73.
10. Kuroda Y, Ooto S, Yamashiro K, et al. Increased choroidal vascularity in central serous chorioretinopathy quantified using swept-source optical coherence tomography. Am J Ophthalmol. 2016;169:199-207.
11. Dansingani KK, Balaratnasingam C, Klufas MA, Sarraf D, Freund KB. Optical coherence tomography angiography of shallow irregular pigment epithelial detachments in pachychoroid spectrum disease. Am J Ophthalmol. 2015;160:1243-1254.
12. Khan S, Engelbert M, Imamura Y, Freund KB. Polypoidal choroidal vasculopathy: simultaneous indocyanine green angiography and eye-tracked spectral domain optical coherence tomography findings. Retina. 2012;32:1057-6108. 13. Fung AT, Yannuzzi LA, Freund KB. Type 1 (sub-retinal pigment epithelial) neovascularization in central serous chorioretinopathy masquerading as neovascular age-related macular degeneration. Retina. 2012;32:1829-1837.
14. Sasahara M, Tsujikawa A, Musashi K, et al. Polypoidal choroidal vasculopathy with choroidal vascular hyperpermeability. Am J Ophthalmol. 2006;142:601-607.
15. Kim JH, Kang SW, Kim TH, Kim SJ, Ahn J. Structure of polypoidal choroidal vasculopathy studied by colocalization between tomographic and angiographic lesions. Am J Ophthalmol. 2013;156:974-980.
16. Nakashizuka H, Mitsumata M, Okisaka S, et al. Clinicopathologic findings in polypoidal choroidal vasculopathy. Invest Ophthalmol Vis Sci. 2008;49):4729-4737.
17. Gal-Or O, Dansingani KK, Sebrow D, Dolz-Marco R, Freund KB. Inner choroidal flow signal attenuation in pachychoroid disease: Optical coherence tomography angiography. Retina. 2018 Jan 30 [Epub ahead of print]
18. Venkatesh P, Takkar B, Temkar S. Clinical manifestations of pachychoroid may be secondary to pachysclera and increased scleral rigidity. Med Hypotheses. 2018;113:72-73.
19. Miyake M, Tsujikawa A, Yamashiro K, et al. Choroidal neovascularization in eyes with choroidal vascular hyperpermeability. Invest Ophthalmol Vis Sci. 2014;55:32233230.
20. Koh A, Lai TYY, Takahashi K, et al. Efficacy and safety of ranibizumab with or without verteporfin photodynamic therapy for polypoidal choroidal vasculopathy: A randomized clinical trial. JAMA Ophthalmol. 2017;135:1206-1213.
21. Erikitola OC, Crosby-Nwaobi R, Lotery AJ, Sivaprasad S. Photodynamic therapy for central serous chorioretinopathy. Eye (Lond). 2014;28:944-957.
22. Padron-Perez N, Arias L, Rubio M, et al. Changes in choroidal thickness after intravitreal injection of anti-vascular endothelial growth factor in pachychoroid neovasculopathy. Invest Ophthalmol Vis Sci. 2018;59:1119-1124. 23.MatsumotoH,HiroeT,MorimotoM,MimuraK,ItoA,Akiyama H. Efficacy of treat-and-extend regimen with aflibercept for pachychoroid neovasculopathy and Type 1 neovascular agerelated macular degeneration. Jpn J Ophthalmol. 2018;62:144150.




