
 |
Cell therapy has the potential to treat a diverse set of refractory medical conditions. It has already been integrated into many medical disciplines and has proven to be efficacious in the regular management of various hematologic diseases.1–3
Multiple clinical trials are investigating cell therapy for ophthalmologic conditions, including age-related macular degeneration, diabetic retinopathy, hereditary retinal degenerations and retinal vein occlusions, according to a search on ClinicalTrials.gov.4 Some trials have already been completed.
However, commercial “cell therapy” clinics threaten to undermine both scientific progress and public trust in stem-cell research. This article explores the state of legitimate research into cell therapy treatments for retinal diseases as well as the scope of the less credible commercial “cell therapy” clinics.
Scientifically rigorous studies
Recently, promising outcomes from scientifically rigorous studies have demonstrated safety and positive anatomic and visual changes following subretinal transplantation of human embryonic stem cell-derived retinal pigment epithelium cells for Stargardt’s macular dystrophy and AMD.5 This safety profile of stem-cell therapy has been paralleled by other published studies, with both dissociated cells and stem cell-derived RPE sheets.6–9
Studies implanting subretinal stem cell-derived RPE sheets in eyes with neovascular and non-neovascular AMD have demonstrated stabilization of vision loss, with a few patients even exhibiting improvement in vision.7–9 Optical coherence tomography imaging has revealed changes consistent with monolayer and host photoreceptor integration.9
Comparably, the subretinal delivery of a single dose of human umbilical stem cells in eyes with geographic atrophy secondary to AMD had no episodes of immune rejection or tumor formation and showed some improvement in visual acuity.6 A relatively high rate of retinal perforation and detachments was attributed to the trans-scleral delivery technique.
Studies have shown early efficacy, stability and functional improvement without significant adverse effects. These results have fortified optimism not only for the research community, but also for patients with progressive, end-stage retinal diseases. Meticulous scientific studies of cell therapies continue to provide evidence to propel these treatments forward.
 |
| The exterior of the U.S. Stem Cell Clinic office in Sunrise, Fla., which was the subject of a warning letter from the Food and Drug Administration and of a New England Journal of Medicine article that reported on three patients who went blind after treatments. (Jim Rassol / South Florida Sun Sentinel / Polaris). |
‘Cell therapy’ clinics gain foothold
However, in recent years commercial “cell therapy” clinics have advertised treatments for a multitude of diseases, including ocular conditions, although none of these clinics have been approved by the Food and Drug Administration.
Access to these clinics is readily available to patients through direct-to-consumer online marketing. Some of these clinics list trials on Clinicaltrials.gov. However, most patients are unaware that Clinicaltrials.gov is a repository for ongoing clinical trials rather than an endorsement of specific clinical trial studies.
The concern of these “cell therapy” clinics targeting various medical conditions has gained international attention, especially following reports of major complications. They include glioproliferative lesions of the spinal cord after intrathecal stem-cell infusions in China, Argentina and Mexico.10
“Cell therapy” clinics are not limited to outside the United States. A 2016 study found 187 U.S. websites offering cell therapy for various diseases at 215 different clinics.11 A similar study found 351 companies in the United States with as many as 570 clinics that market cell therapy direct to consumers.12
 |
Our study of commercial clinics
We performed a study to assess U.S.-based companies with websites that promoted online, direct-to-consumer access to “cell therapies” for ocular disease. From our search, we discovered approximately 40 companies that do so for ophthalmic conditions at 76 clinics across the country.
These clinics offer interventions for a wide range of ocular conditions, most commonly macular degeneration, optic neuritis, retinitis pigmentosa and diabetic retinopathy. In regards to the source of “cell therapy” they use, the overwhelming majority are autologous-based treatments, with a small number offering allogenic-based treatments.
The most frequently advertised “cell therapy” source is autologous adipose-derived stem cells, while less common sources include autologous bone marrow-derived stem cells, amniotic stem cells, peripheral blood-derived stem cells, umbilical cord blood stem cells, placental stem cells, allogenic bone marrow-derived stem cells and xenocells.
 |
Where’s the evidence?
Most companies did not advertise the specific modes of “cell therapy” delivery. Among those that did, the most common route of administration was intravenous. Ocular-specific “cell therapy” delivery processes include “eye injection,” intravitreal injection, retrobulbar injection, eye drops, retrofundal injection, sub-Tenon’s injection and intraocular injection with vitrectomy. The cost for these treatments ranged from $4,000 to $10,500, although some reports have listed prices upwards of $50,000.13
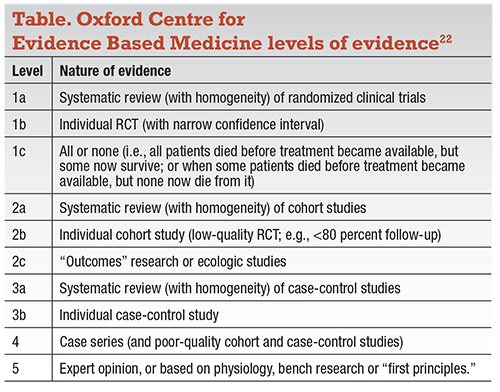
We also studied the level of scientific evidence for treatments at these clinics for retinal diseases. We performed a PubMed search and classified publications from these “cell therapy” clinics using the Oxford Centre Level of Evidence (Table). We found a paucity of publications supporting these treatments: no level 1, 2, 3 or 4 evidence. There was one case report (level 5 evidence) of a patient with serpiginous choroidopathy who was treated with intravitreal bone marrow-derived stem cells and was reported to have an improvement in vision.14
Given the paucity of publications from commercial cell therapy clinics, we examined their websites to see whether they advertised any evidence of their treatments. Although we didn’t find information about numbers of patients treated and specific outcomes, nearly half (43 percent) of the websites suggested or explicitly claimed “clinically significant benefits” from their treatments.
Thirty percent claimed that their treatments would be better than the standard of care for conditions such as macular degeneration. Nearly half (43 percent) of the websites did not list any risks from the treatments, and 20 percent listed only “minor risks.” Furthermore, only half of the websites acknowledged that these treatments are experimental.
 |
Disastrous outcomes
The positive outcomes with minimal risks touted by the cell therapy clinic websites are in stark contrast to the re- ports of severe, blinding complications from their treatments.
The most notable publication to date is a case series of three patients with macular degeneration who were treated at a single clinic in Florida with bilateral intravitreal injections.15 Five of the six treated eyes went on to develop retinal detachments with proliferative vitreoretinopathy.15 The vision in those patients deteriorated from 20/30 to 20/50 in their better-seeing eye before treatment to 20/200 to no light perception afterward.
Another case report described a pa- tient with macular degeneration who had bilateral intravitreal injections at a different clinic and then developed bi- lateral retinal detachments with severe proliferative vitreoretinopathy.16 The pa- tient’s vision worsened from 20/400 to hand motion in the right eye and 20/200 to LP in the left.
A subsequent case report involved a patient with Stargardt’s disease who received a subretinal injection of bone marrow-derived stem cells and developed a retinal detachment.17 The patient’s vision deteriorated from 20/400 to HM before having RD repair, which restored his vision to his baseline.
Call for increased regulation
The severity of these complications points to a need for increased regulation of these clinics. They claim to bypass FDA regulation by reasoning that cells are minimally manipulated and are utilized for homologous use.
The FDA responded by issuing draft guidance statements in December 2014 and October 2015, updated in December 2017, defining the term “minimally manipulated” stem cells to delineate homologous use.18,19 This was done to delineate that autologous stem cells and their application fall under the regulatory oversight of the FDA.
 |
In 2016 the American Academy of Ophthalmology issued a clinical statement underscoring that no FDA-approved stem-cell therapies exist for ocular conditions, and that the risks associated with such treatments are unknown.20 Due to the concern for lack of evidence of safety and tissue handling, the FDA has begun issuing warnings to various clinics in an effort to address this problem.21
Patients need education
Although cell therapy has the potential to have a significant impact on retinal diseases, it’s important that patients understand the current limitations of treatment and receive education about the potential complications from treatments at these commercial “cell therapy” clinics.
Patients should be educated to avoid “cell therapies” that require out-of-pocket payment, offer bilateral treatments, and are done by centers that provide only “cell therapy” treatments.
Legitimate scientific cell-therapy clinical trials are typically sponsored by either the government or companies and don’t ask for payment out-of-pocket. Legitimate clinical trials don’t perform bilateral simultaneous treatments until safety has been demonstrated. Lastly, these scientific studies are carried out by retina specialists who can provide other treatments for early stage disease, such as anti-VEGF for exudative macular degeneration. RS
REFERENCES
1. Mittal P, Saliba RM, Giralt SA, et al. Allogeneic transplantation: a therapeutic option for myelofibrosis, chronic myelomonocytic leukemia and Philadelphia- negative/BCR-ABL-negative chronic myelogenous leukemia. Bone Marrow Transplant. 2004;33:1005-1009.
2. Ditschkowski M, Beelen DW, Trenschel R, Koldehoff M, Elmaagacli AH. Outcome of allogeneic stem cell transplantation in patients with myelofibrosis. Bone Marrow Transplant. 2004;34:807-813.
3. Ballen KK, Shrestha S, Sobocinski KA, et al. Outcome of transplantation for myelofibrosis. Biol Blood Marrow Transplant J Am Soc Blood Marrow Transplant. 2010;16:358-367.
4. ClinicalTrials.gov. https://clinicaltrials.gov/. Accessed July 14, 2018.
5. Schwartz SD, Regillo CD, Lam BL, et al. Human embryonic stem cell- derived retinal pigment epithelium in patients with age-related macular degeneration and Stargardt’s macular dystrophy: follow-up of two open- label phase 1/2 studies. Lancet. 2015;385:509-516.
6. Ho AC, Chang TS, Samuel M, Williamson P, Willenbucher RF, Malone T. Experience with a subretinal cell-based therapy in patients with geographic atrophy secondary to age-related macular degeneration. Am J Ophthalmol. 2017;179:67-80.
7. Cruz L da, Fynes K, Georgiadis O, et al. Phase 1 clinical study of an embryonic stem cell–derived retinal pigment epithelium patch in age-related macular degeneration. Nat Biotechnol. 2018;36:328-337.
8. Mandai M, Watanabe A, Kurimoto Y, et al. Autologous Induced stem- cell–derived retinal cells for macular degeneration. N Engl J Med. 2017;376:1038-1046.
9. Kashani AH, Lebkowski JS, Rahhal FM, et al. A bioengineered retinal pigment epithelial monolayer for advanced, dry age-related macular degeneration. Sci Transl Med. 2018;10:eaao4097.
10. Berkowitz AL, Miller MB, Mir SA, et al. Glioproliferative lesion of the spinal cord as a complication of “stem-cell tourism.” N Engl J Med. 2016;375:196- 198.
11. Berger I, Ahmad A, Bansal A, Kapoor T, Sipp D, Rasko JEJ. Global distribution of businesses marketing stem cell-based interventions. Cell Stem Cell. 2016;19:158-162.
12. Turner L, Knoepfler P. Selling Stem Cells in the USA: Assessing the direct- to-consumer industry. Cell Stem Cell. 2016;19:154-157.
13. Taylor-Weiner H, Graff Zivin J. Medicine’s wild west-unlicensed stem-cell clinics in the United States. N Engl J Med. 2015;373:985-987.
14. Weiss JN, Levy S Benes SC. Stem Cell Ophthalmology Treatment Study (SCOTS): Bone marrow-derived stem cells in the treatment of Leber’s hereditary optic neuropathy. Neural Regen Res. 2016;11:1685-1694.
15. Kuriyan AE, Albini TA, Townsend JH, et al. Vision loss after intravitreal injection of autologous “stem cells” for AMD. N Engl J Med. 2017;376:1047- 1053.
16. Saraf SS, Cunningham MA, Kuriyan AE, et al. Bilateral retinal detachments after intravitreal injection of adipose-derived “stem cells” in a patient with exudative macular degeneration. Ophthalmic Surg Lasers Imaging Retina. 2017;48:772-775.
17. Leung EH, Flynn HW, Albini TA, Medina CA. Retinal detachment after subretinal stem cell transplantation. Ophthalmic Surg Lasers Imaging Retina. 2016;47:600-601.
18. Homologous use of human cells, tissues, and cellular and tissue-based products. Draft guidance for industry and Food and Drug Administration staff. Silver Spring, MD: FDA; October 2015. https://www.govinfo.gov/content/ pkg/FR-2015-10-30/html/2015-27704.htm. Accessed February 12, 2019.
19. Minimal manipulation of human cells, tissues and cellular and tissue-based products: Draft guidance for industry and Food and Drug Administration staff. Silver Spring, MD: FDA; December 2014. https://www.govinfo.gov/content/ pkg/FR-2014-12-23/html/2014-30011.htm. Accessed February 12, 2019.
20. Intraocular Stem Cell Therapy–2016. American Academy of Ophthalmology. Updated June 2016. Available at: https://www.aao.org/ clinical-statement/intraocular-stem-cell-therapy. Accessed July 14, 2018.
21. US Food and Drug Administration. Warning letter to US Stem Cell Clinic, LLC. August 24, 2017. Available at: https://www.fda.gov/ICECI/ EnforcementActions/WarningLetters/2017/ucm573187.htm. Accessed July 15, 2018.
22. Oxford Centre for Evidence-based Medicine—Levels of Evidence (March 2009. Center for Evidence Based Medicine. https://www.cebm.net/2009/06/ oxford-centre-evidence-based-medicine-levels-evidence-march-2009/ Accessed December 14, 2018.



