 |
Thus, we hypothesized that decreasing the outflow via a potent aqueous suppressant such as dorzolamide hydrochloride-timolol maleate may effectively reduce the clearance of the intravitreal drug.7 Here, we report on a small study we conducted that aimed to evaluate the effects of adding topical dorzolamide-timolol (Cosopt, Akorn, Lake Forest, Ill.) to a fixed-interval intravitreal anti-VEGF regimen on both the anatomic and functional outcomes in patients with neovascular AMD who had not responded optimally.
We found that in all 10 patients (10 eyes) who completed the study, mean central subfield thickness (CST) decreased from 419.7 μm at enrollment to 334.1 μm at the final visit (P=0.01), and we noted decreases as soon as the first visit after enrollment. We found all had a reduced CST on the final visit after instilling the combination therapy (Table 1).
Methodology
Our
 |
The study excluded patients who had a history of uveitis, pars plana vitrectomy, glaucoma surgery, any eye surgery conducted six months prior to enrollment, a history of either anti-glaucoma therapy or sulphonamide allergy, current use of diuretics or corticosteroids and systemic contraindications to topical β-blocker therapy. Enrolled patients continued to receive intravitreal injections with the same anti-VEGF drug and fixed-interval regimen that they had been receiving, with the only change being the addition of topical dorzolamide-timolol.
We then instructed the patients to instill the eye drops twice daily in the study eye for the duration of the study period. We assessed their progress by measuring the CST, and gathered additional measurements such as maximum subretinal fluid (SRF) height and maximum pigment epithelial detachment (PED) height for analysis using SD-OCT imaging.
 |
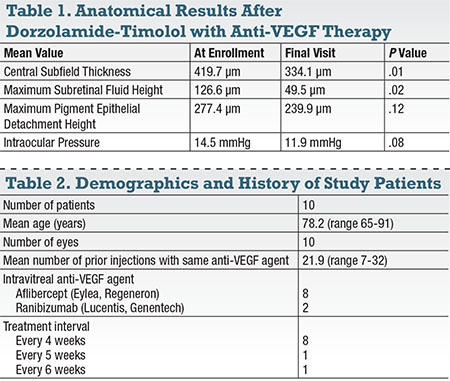
| Figure 1. The study design called for patients to be on the same anti-VEGF drug with the same fixed interval between injections for at least two visits prior to enrollment. Following enrollment (dark blue), patients were maintained on the same anti-VEGF drug with the same interval between injections for the study duration. The only addition was topical dorzolamide-timolol BID. While the endpoint was planned at the second visit, eight of 10 eyes stayed on the drops through a third study visit. |
At each encounter, we assessed visual acuity (VA) and intraocular pressure (IOP) using the best available Snellen visual acuity and tonometry, respectively. Analysis was subsequently conducted using the paired t-test (GraphPad, GraphPad Software Inc., La Jolla, Calif.) in order to assess the changes in the previously mentioned parameters, with a statistically significant threshold of P<0.05. Figure 1 summarizes the study design.
Results
Ten patients (10 study eyes) completed the study protocol and were included for the final analysis. Mean CST decreased from 419.7 μm at enrollment to 334.1 μm at the final visit (P=0.01), with a decrease noted as soon as the first visit after enrollment. All patients were found to have a reduced CST on the final visit after instilling the combination therapy.
In addition to the decrease in CST (Figure 2), mean maximum SRF height also decreased from 126.6 μm at enrollment to 49.5 μm at the final visit (P=0.02) with the decrease also noted at the first visit after enrollment (Figure 3). Moreover, all study eyes experienced a decrease in maximum SRF height at the final visit. Additionally, four out of the 10 study eyes demonstrated complete resolution of SRF by the final visit.
Mean maximum
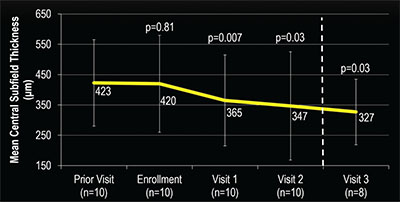 |
| Figure 2. Change in the mean central subfield thickness (CST) after administering anti-VEGF and dorzolamide-timolol in study eyes was fairly stable for the visit prior to enrollment vs. the enrollment visit. However, CST showed a significant decrease as soon as the first visit after starting dorzolamide-timolol that continued throughout the duration of the study. |
How The Eye Clears Drugs
Reports have estimated that 15 percent of patients who suffer from neovascular AMD are classified as non-responders.1,8 A variety of treatment regimens have been tried in these patients in the past. The combination of intravitreal anti-VEGF and topical dorzolamide-timolol is a novel approach that appears to provide some benefit for such patients. We believe that the therapy works by primarily delaying the outflow of the anti-VEGF drug.3,7
While no concrete proof has shown how the eye clears anti-VEGF agents, some evidence has supported the premise that aqueous outflow to an extent removes the drugs.4,5 In one study, the rate of drug clearance from the vitreous humor paralleled the rate of clearance from the aqueous humor, which reinforces the hypothesis of a common outflow.6 We already know that dorzolamide-timolol is a powerful aqueous suppressant, decreasing production by up to 50 percent.7 Perhaps decreasing aqueous production consequently reduces aqueous (and drug) outflow.
One impetus for our study that supports this hypothesis was a study of patients with macular edema due to retinal vein occlusion.3 In this study, patients receiving a single injection of bevacizumab were randomly assigned to receive
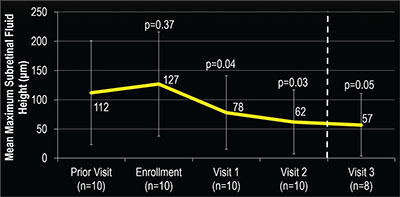 |
| Figure 3. Mean maximum subretinal fluid (SRF) height after administering anti-VEGF and dorzolamide-timolol in study eye was fairly stable in the visit prior to enrollment and the enrollment visit. However, a significant decrease in SRF height was seen as soon as the first visit after starting dorzolamide-timolol that continued throughout the study. |
It is also possible that the β-blocker or the carbonic anhydrase inhibitor itself may have led to the positive responses in our study. β-blockers, for example, have been shown to reduce upregulation of angiogenic factors via β2-andregenic receptor blockade9,10 and attenuation of choroidal neovascularization.11
One
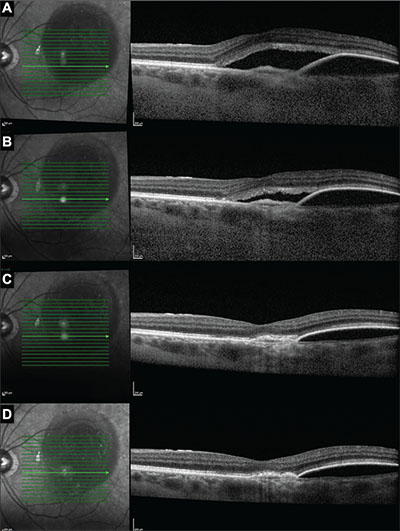 |
| Figure 4. Optical coherence tomography images in one subject at enrollment (A), week four (B), week eight (C) and week 12 (D) show improvement in subretinal fluid and pigment epithelial detachment after the addition of dorzolamide-timolol to the monthly aflibercept regimen. Prior to enrollment, this patient had received 16 aflibercept injections. |
Dorzolamide has also been used in the successful treatment of macular edema in a variety of cases.15-18 The carbonic anhydrase enzyme has been found in both Müller cells and retinal pigment epithelial cells,19,20 and the inhibition of the enzyme may modulate the pump function in these cells, leading to fluid egress from the retina to the choroid. Dorzolamide has also been shown to increase choroidal perfusion and retinal oxygenation,21,22 which might suggest an independent role of the drug in reducing macular edema.
Study Limitations
Two
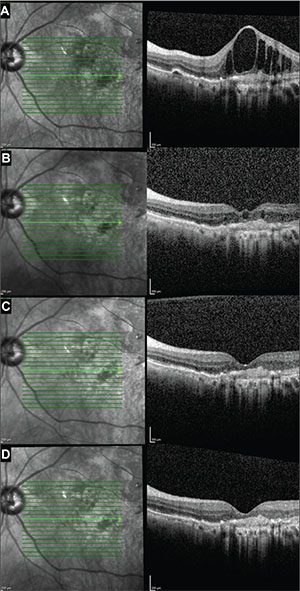 |
| Figure 5. Optical coherence tomography images in a second subject at enrollment (A), week four (B), week eight (C) and week 12 (D) show rapid improvement in the intraretinal edema after the addition of dorzolamide-timolol to the monthly aflibercept regimen. Prior to enrollment, this patient had received 30 aflibercept injections. |
Additionally, the study design does not allow us to determine whether the observed effects were due to the independent action of dorzolamide vs. timolol rather than the combination of dorzolamide-timolol. Another limitation was the lack of a control group and the possibility that our findings represent normal fluctuations of the disease. However, this is unlikely as we only included patients who had persistent exudation over six months prior to inclusion despite fixed-interval anti-VEGF injections. In fact, the mean CST, SRF and PED heights at the visit prior to enrollment
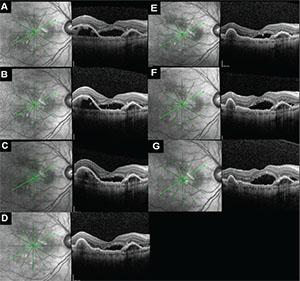 |
| Figure 6. After the addition of dorzolamide-timolol to monthly ranibizumab regimen, optical coherence tomography (OCT) images in another subject at enrollment (A), week four (B), week eight (C), week 12 (D) and week 16 (E) show a gradual decrease in subfoveal subretinal fluid (SRF) as well as pigment epithelial detachment on the left side of the images. Dorzolamide-timolol was discontinued after week 16 and the patient continued to receive monthly intravitreal ranibizumab only. The subsequent OCT images at week 20 (F) and week 24 (G) demonstrate increasing SRF after stopping the drop. Prior to enrollment, this patient had received 30 ranibizumab injections. |
Patient compliance with the eye drop presented another limitation because the study measured it via self-reporting. However, the consistent drop in intraocular pressure amongst the study eyes suggests, to a certain extent, compliance.
What’s Next
 |
The use of dorzolamide-timolol as an adjuvant therapy to intravitreal anti-VEGF agents is a potentially effective regimen in reducing edema and subretinal fluid in eyes that suffer from neovascular AMD and do not respond completely to traditional anti-VEGF treatment. While our study did not show any vision changes, the possibility exists that visual acuity may improve with earlier initiation of the drops and longer-term therapy.
Recently, we performed an analysis of the data set from the Comparison of AMD Treatment Trial (CATT)26 exploring the effect of aqueous suppressants on outcomes. We will present these findings in the near future. A larger, multicenter sham-controlled randomized study with a larger sample size is about to start enrolling; it will seek to determine whether dorzolamide-timololtruly has a beneficial effect on the subset of neovascular AMD patients with persistent exudation despite consistent anti-VEGF injections. RS
REFERENCES
1. Krebs I, Glittenberg C, Ansari-Shahrezaei S, Hagen S, Steiner I, Binder S. Non-responders to treatment with antagonists of vascular endothelial growth factor in age-related macular degeneration. Br J Ophthalmol. 2013;97:1443-1446.
2. Barthelmes D, Walton R, Campain AE, et al; Fight Retinal Blindness! Project Investigators. Outcomes of persistently active neovascular age-related macular degeneration treated with VEGF inhibitors: observational study data. Br J Ophthalmol. 2015;99:359-364.
3. Byeon SH, Kwon OW, Song JH, Kim SE, Park YS. Prolongation of activity of single intravitreal bevacizumab by adjuvant topical aqueous depressant (timolol-dorzolamide). Graefes Arch Clin Exp Ophthalmol. 2009;247:35-42.
4. Bakri SJ, Snyder MR, Reid JM, Pulido JS, Singh RJ. Pharmacokinetics of intravitreal bevacizumab (Avastin). Ophthalmology. 2007;114:855-859.
5. Stewart MW. Predicted biologic activity of intravitreal bevacizumab. Retina. 2007;27:1196-1200.
6. Gaudreault J, Fei D, Rusit J, Suboc P, Shiu V. Preclinical pharmacokinetics of ranibizumab (rhuFabV2) after a single intravitreal administration. Invest Ophthalmol Vis Sci. 2005;46:726-733.
7. Brubaker RF, Ingram CJ, Schoff EO, Nau CB. Comparison of the efficacy of betaxolol-brinzolamide and timolol-dorzolamide as suppressors of aqueous humor flow in human subjects. Ophthalmology. 2000;107283-287.
8. Ersoy L, Ristau T, Kirchhof B, Liakopoulos S. Response to anti-VEGF therapy in patients with subretinal fluid and pigment epithelial detachment on spectral-domain optical coherence tomography Graefes Arch Clin Exp Ophthalmol. 2014;252: 889-897.
9. Martini D, Monte MD, Ristori C, et al. Antiangiogenic effects of ß2-adrenergic receptor blockade in a mouse model of oxygen-induced retinopathy. J Neurochem. 2011;119:1317-1329.
10. Casini G, Dal Monte M, Fornaciari I, Filippi L, Bagnoli P. The ß-adrenergic system as a possible new target for pharmacologic treatment of neovascular retinal diseases. Prog Retin Eye Res. 2014;42:103-129.
11. Lavine JA, Sang Y, Wang S, Ip MS, Sheibani N. Attenuation of choroidal neovascularization by ß2-adrenoreceptor antagonism. JAMA Ophthalmology. 2013;131(3):376-382.
12. Montero JA, Ruiz-Moreno JM, Sanchis-Merino E, Perez-Martin S. Systemic ß-blockers may reduce the need for repeated intravitreal injections in patients with wet age-related macular degeneration treated by bevacizumab. Retina. 2013;33:508-512.
13. Klein R, Myers CE, Klein BEK. Vasodilators, blood pressure–lowering medications, and age-related macular degeneration: the Beaver Dam Eye Study. Ophthalmology. 2014;121:1604-1611.
14. Thomas AS, Redd T, Hwang T. Effect of systemic ß-blockers, ACE inhibitors, and angiotensin receptor blockers on development of choroidal neovascularization in patients with age-related macular degeneration. Retina. 2015;35:1964-1968.
15. Grover S, Apushkin MA, Fishman GA. Topical dorzolamide for the treatment of cystoid macular edema in patients with retinitis pigmentosa. Am J Ophthalmol. 2006;141:850-858.
16. Genead MA, McAnany JJ, Fishman GA. Topical dorzolamide for treatment of cystoid macular edema in patients with choroideremia. Retina. 2012;32:826-833.
17. Genead MA, Fishman GA. Efficacy of sustained topical dorzolamide therapy for cystic macular lesions in patients with retinitis pigmentosa and Usher syndrome. Arch Ophthalmol. 2010;128:1146-1150.
18. Genead MA, Fishman GA, Walia S. Efficacy of sustained topical dorzolamide therapy for cystic macular lesions in patients with X-linked retinoschisis. Arch Ophthalmol. 2010;128 190-197.
19. Terashima H, Suzuki K, Kato K, Sugai N. Membrane-bound carbonic anhydrase activity in the rat corneal endothelium and retina. Jpn J Ophthalmol. 1996;40:142-153.
20. Adijanto J, Banzon T, Jalickee S, Wang NS, Miller SS. CO2-induced ion and fluid transport in human retinal pigment epithelium. J Gen Physiol. 2009;133:603-622.
21. Harris A, Jonescu-Cuypers CP, Kagemann L, et al. Effect of dorzolamide timolol combination versus timolol 0.5% on ocular blood flow in patients with primary open-angle glaucoma. Am J Ophthalmol. 2001;132:490-495.
22. Harris A, Ciulla TA, Pratt LM, et al. The effects of dorzolamide on choroidal and retinal perfusion in non-exudative age related macular degeneration. Br J Ophthalmol. 2003;87:753-757.
23. Zhu M, Chew JK, Broadhead GK, et al. Intravitreal ranibizumab for neovascular age-related macular degeneration in clinical practice: five-year treatment outcomes. Graefes Arch Clin Exp Ophthalmol. 2015;253:1217-1225.
24. Amoaku WM, Chakravarthy U, Gale R, et al. Defining response to anti-VEGF therapies in neovascular AMD. Eye (Lond). 2015;29:721-731.
25. Hanout M, Horan N, Do DV. Introduction to microperimetry and its use in analysis of geographic atrophy in age-related macular degeneration. Curr Opin Ophthalmol. 2015;26:149-156.
26. The CATT Research group. Ranibizumab and bevacizumab for treatment of neovascular age-related macular degeneration: two-year results. Ophthalmology 2012;119:1388-98.



