 |
Until recently, the burden, definition, clinical features and natural course of this debilitating condition have been poorly understood, but long-term, follow-up clinical studies and advances in ocular imaging have enabled significant progress in our understanding of the disease mechanisms and natural history of MMD. The following imaging modalities have contributed to our knowledge of MMD:
• Spectral-domain optical coherence tomography (SD-OCT) has enabled the visualization of the retinal pigment epithelium and Bruch’s membrane.
• Swept-source OCT (SS-OCT) has provided greater resolution for the imaging of the deep choroid and sclera in pathologic myopia.9
• OCT angiography (OCT-A) has further allowed for the noninvasive imaging of the retinal and choroidal circulation and has provided new insights into MMD pathogenesis.10
• Widefield angiography has revealed vascular anomalies in the highly myopic eye.
• Widefield fluorescein angiography has captured areas of nonperfusion in the peripheral retina.
• Widefield indocyanine green angiography has demonstrated the posterior migration of vortex veins.11,12
• Widefield fundus photography and widefield OCT will likely set future standards for imaging of posterior staphyloma in highly myopic eyes.13,14
In this review, we describe the definition, pathogenesis, clinical features and imaging aspects of and potential treatment options for MMD.
Definition and Classification
Earlier studies on the epidemiology of MMD were hampered by a lack of both a consistent definition and a unified classification. Several classifications and definitions existed, but none of them considered the natural history and progression of MMD.15-17 The lack of a common classification made it difficult to perform direct comparison or pooling of
data from different studies to evaluate the prevalence, incidence and risk factors for MMD.
To resolve this issue, the META-analysis for Pathologic Myopia (META-PM) group, proposed a classification system of MMD in five categories (Table):
• 0, no myopic retinal lesions;
• 1, tessellated fundus only;
• 2, diffuse chorioretinal atrophy;
• 3, patchy chorioretinal atrophy; and
• 4, macular atrophy.
These categories were defined based on long-term clinical observation that informed on the progression patterns and risk of myopic choroidal neovascularization development for each stage.
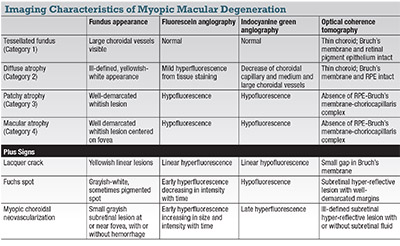 |
Three additional features, observed to occur in any category of MMD, were included as “plus signs:”
• lacquer cracks;
• mCNV; and
• Fuchs spot.
This classification system has been adopted in recent studies and has shown good intraand inter-grader reliability.10,18,19
Rapidly Escalating Incidence
The prevalence of high myopia and related visual impairment is rapidly escalating. A systematic review and meta-analysis of the prevalence of myopia and high myopia found an estimated 163 million people with high myopia (2.7 percent of the world population) in the year 2000. This number is predicted to grow to 938 million (9.8 percent of the world population) by the year 2050.2
 |
Another report estimated that 10 million people suffered from MMD-related visual impairment in 2015 (prevalence 0.13 percent, 95 percent confidence interval [CI] 5.5 to 23.7 million, 0.07 to 0.34 percent), 3.3 million of whom were blind (0.04 percent, 1.8 to 7.8 million, CI 0.03 to 0.10 percent).
By 2050, 55.7 million people will be visually impaired as a result of MMD (0.57 percent, 29 to 119.7 million, 95 percent CI 0.33 to 1.11 percent). Among them, 18.5 million will be blind (0.19 percent, 9.6 to 39.7 million, 95 percent CI 0.11 to 0.37 percent).20
Recent population-based studies have used the META-PM classification for myopic maculopathy to analyze the incidence and progression of myopic maculopathy.
In the Handan Eye Study (n=5,394), the five-year incidence of myopic maculopathy was 0.05 percent (95 percent CI 0.02 to 0.10 percent) and progression occurred in 35.3 percent.21 The Beijing Eye Study (n=4,439) found a similar progression rate of 35.5 percent, but over a longer 10-year period.22 In the Chinese American Eye Study, the prevalence of any MMD was 32.2 percent, and was higher among older subjects, those with more severe myopia and longer axial length.23 Data from the IRIS registry and the National Health and Nutrition Examination Survey (NHANES) indicated a diopter-adjusted prevalence of high myopia, progressive high myopia and mCNV of 3.92 percent.24
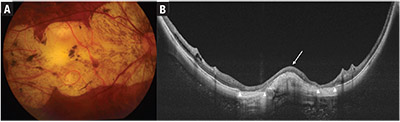 |
| Figure 1. Fundus photograph and swept-source optical coherence tomographic (SS-OCT) scan of the left eye of a patient with Category 4 myopic maculopathy. Fundus photograph (A) shows a large, well-defined yellowish-white lesion involving the fovea and most of the macula, representing confluent areas of patchy chorioretinal atrophy. Choroidal and scleral vessels can be seen coursing through atrophic areas. The SS-OCT scan (B) shows a dome-shaped macula (arrow) with loss of the choroid-Bruch’s membrane-retinal pigment epithelium complex on either side of the dome. Increased transmission of light signal (arrow heads) into the sclera and orbital fat can be seen corresponding to areas of complete loss of the retinal pigment epithelium. |
The table describes the imaging features of MMD under the META-PM classification.
• Tessellated fundus (Category 1) represents the most subtle and earliest clinical features of MMD.
In the tessellated fundus, choroidal vessels can be observed clearly around the fovea as well as around the retinal vascular arcade on fundus photography. The choroid appears thin on OCT but the outer retina, RPE and Bruch’s membrane are intact.1
• Diffuse chorioretinal atrophy (Category 2) presents as an ill-defined yellowish-white appearance of the posterior pole on fundus photography. The atrophy usually begins in the temporal peripapillary region and increases with age to involve the posterior pole. On fundus fluorescein angiography, mild hyperfluorescence from tissue staining is seen in the late phase. On indocyanine green angiography, a marked decrease of the choroidal capillary and medium and large-sized choroidal vessels has been described within the area of diffuse atrophy.25 OCT shows marked choroidal thinning, but the outer retinal layers and RPE remain intact.
OCT-A has shown variability in the degree of choriocapillaris flow-signal loss, ranging from no apparent loss to extensive loss with unmasking of underlying large choroidal vessels.10
• Patchy chorioretinal atrophy (Category 3) (Figure 1) is characterized by a complete absence of the choriocapillaris, eventually progressing to a loss of the outer retina and retinal pigment epithelium, although the inner retina remains largely intact. Clinically, patchy chorioretinal atrophy appears as clearly demarcated, lobular grayish-white lesions in the macula, representing areas in which the sclera can be seen through the transparent retina. Pigment clumping may be observed within the area of patchy atrophy.
On autofluorescence imaging, these areas show hypoautofluorescence from the loss of RPE. The lesion appears hypofluorescent on FA and ICGA due to choroidal filling defect. Absence of the retinal pigment epithelium can be identified as areas of increased transmission of signal into the underlying sclera on OCT, allowing clear visualization of the scleral contour, episclera and orbital fat. The entire choroid, with the exception of large choroidal vessels, is absent. These areas may be associated with loss of the outer retina with a bridge of inner retina, but in most instances the inner retina can be seen to rest directly on the scleral bed. Occasionally, the inner retina may herniate into the sclera, presumably in areas of scleral ectasia.25,26
• Macular atrophy (Category 4) is a clearly demarcated, grayish-white or whitish, atrophic chorioretinal lesion centered on the fovea. The imaging characteristics are similar to those of patchy chorioretinal atrophy. A well-defined subretinal hyper-reflective lesion representing the fibrotic remains of a regressed CNV may sometimes appear on OCT. The majority of macular atrophy develops from mCNV, with only a small percentage related to expansion of patchy atrophy.26,27
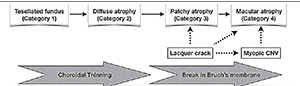 |
| Figure 2. Progression of myopic macular degeneration (MMD) reveals increasing thinning of the choroid from tessellated fundus (Category 1) to diffuse chorioretinal atrophy (Category 2). Bruch’s membrane breaks trigger progression to the late stages of myopic macular degeneration. Patchy chorioretinal atrophy (Category 3) develops around lacquer cracks and may progress to patchy related macular atrophy (Category 4), although in most cases, myopic CNV-related macular atrophy is the cause of Category 4 MMD. |
The longest follow-up study to date found a progression rate of 47:1,000 eye-years among 810 eyes of 432 patients in a hospital-based population.27 The progression rate was highest in eyes with macular atrophy at baseline, followed by patchy atrophy, diffuse atrophy and myopic eyes without MMD.
Risk factors for progression were: female gender; longer axial length; greater axial elongation; and development of parapapillary atrophy.
In the earlier stages of MMD, the predominant process is progressive choroidal thinning (Figure 2) from tessellated fundus (Category 1) to diffuse atrophy (Category 2). Diffuse atrophy tends to start in the parapapillary region and expands to involve the macula.
Breaks in Bruch’s membrane are the main drivers for progression to the more severe grades of MMD. These breaks can arise from lacquer cracks or myopic CNV. Patchy atrophy (Category 3) develops around lacquer cracks and may expand to involve the fovea (patchy related macular atrophy, Category 4). Atrophy developing around the site of a regressed myopic CNV is thought to be due to rupture of Bruch’s membrane, and is the main cause of macular atrophy (Category 4).
Plus Signs of MMD
• Lacquer cracks are thought to be mechanical breaks of the Bruch’s membrane. Clinically they appear as yellowish linear lesions, sometimes in a stellate pattern, in the macula. Lacquer cracks are difficult to discern on clinical examination or fundus photography alone. ICGA is widely accepted as the best method for detecting lacquer cracks, which typically appear as linear hypofluorescence in the late phase of ICGA.25 Subretinal bleeding is often observed at the onset of lacquer cracks, but is usually absorbed spontaneously with good visual recovery.
It is worth noting that myopic CNV may develop at sites of lacquer cracks as well; thus, FFA and OCT should be performed in cases of subretinal bleeding when clinical suspicion merits it.25
• mCNV (Figure 3) appears on clinical examination as a small grayish subretinal lesion at or near the fovea, with or without hemorrhage. mCNV evolves from the active stage to scarred stage (Fuchs spot) and, finally, atrophic stage.28 The diagnosis is confirmed on fundus fluorescein angiogram where the lesion demonstrates hyperfluorescence that increases in size and intensity with time, indicating leakage.29
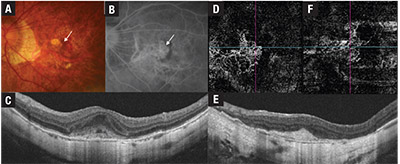 |
| Figure 3. Multimodal imaging of myopic choroidal neovascularization (mCNV) before and after treatment with intravitreal anti-VEGF injections. Fundus photograph (A) shows a dark red patch of subretinal hemorrhage (arrow). Corresponding fundus fluorescein angiogram (B) shows early hyperfluorescence in a lacy pattern in the subfoveal location (arrow). Swept-source optical coherence tomography (C) shows a subretinal hyper- reflective lesion with indistinct margins. A 3-by-3-mm OCT angiography scan (D) through the outer retina segment shows a network of vessels representing the mCNV lesion. After treatment with four anti-VEGF injections, the mCNV lesion (E) has acquired a distinct border and subretinal fluid is absent. Flow signals persist in the inactive mCNV lesion (F), but the vessel network appears to be less dense compared with the pre-treatment OCT-A. |
With treatment, the hyper-reflective lesion consolidates and acquires a distinct border. OCT-A is useful for identifying the choroidal neovascular membrane noninvasively.31,32 However, OCT-A has lower sensitivity compared to FFA, and cannot replace the latter as the diagnostic gold standard.33
Another limitation of OCT-A is the lack of information on disease activity. Flow-signal may persist in an inactive mCNV; therefore OCT and FFA are still required to monitor the level of activity.32-34 mCNV responds remarkably well to treatment with intravitreal anti-VEGF agents, often requiring only a few injections for disease control.35 Chorioretinal atrophy may develop around the CNV lesion, particularly if left untreated, leading to macular atrophy and eventual visual loss.36,37
Studies have revealed that macular atrophy developing after mCNV may be the result of a Bruch’s membrane rupture.36
• Fuchs spot is a grayish-white, sometimes pigmented lesion representing the scar phase of myopic CNV.3 Accordingly, the lesion stains but does not leak on FFA. On OCT, Fuchs spot appears as a subretinal hyper-reflective lesion with sharply delineated borders not associated with exudation.
• Posterior staphyloma (Figure 4) is a key lesion of pathologic myopia. With the advent of SDOCT, Richard Spaide, MD, defined staphyloma as an outpouching of the wall of the eye that has a radius of curvature less than the surrounding curvature of the eye wall itself.38 Clinically, posterior staphyloma can be identified on binocular indirect ophthalmoscopy by an abrupt change in the retinal surface contour posteriorly.
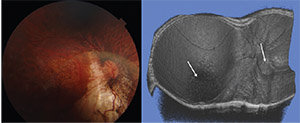 |
| Figure 4. Fundus photograph and swept-source optical coherence tomographic scan of a patient with compound posterior staphyloma. Reconstruction of the 12-by-9-mm scans into a three-dimensional image (right) clearly shows a compound posterior staphyloma with two apices, one at the optic nerve head and another centered on the fovea (arrows). |
Three-dimensional MRI is an effective method of visualizing the posterior staphyloma, but is cumbersome and expensive to perform.
Kyoko Ohno Matsui, MD, employed a combination 3D-MRI and widefield fundus images to characterize the different types of posterior staphyloma, and found that wide macular staphyloma was the most common, followed by narrow macular staphyloma.39 SS-OCT volume scans with a maximum scan width of 12 mm through the macula and optic disc can be reconstructed three-dimensionally and allow some appreciation of the posterior globe contour, although this method is still limited by its scan width. Anthony Kuo, MD, and colleagues, including members of our group, reported that distortion-corrected OCT measurements of the posterior eye radius of curvature and asphericity were comparable to values obtained from 3D-MRI and could potentially be used to study posterior eye shape.40
Widefield OCT may offer an easier way to characterize posterior staphyloma. A multinational study led by Kosei Shinohara, MD, showed that WF-OCT was equivalent to 3D-MRI in detecting posterior staphyloma, and additionally allowed the study of spatial relationships between the staphyloma, the optic nerve and the retinal structures.13
Pathogenesis of MMD
The mechanisms that lead to the development of the macular and retinal changes in MMD remain hypothetical.25 A widely accepted view is the “mechanical” theory of MMD in which axial elongation of the globe from scleral remodeling results in the mechanical stretching of the retina, leading to decreased photoreceptor density and pathologic features such as lacquer cracks and chorioretinal atrophy.41
In addition to the “mechanical” theory, some authors have suggested that choroidal thinning, leading to decreased choroidal perfusion and ischemia and subsequent upregulation of angiogenic factors in some eyes, may also be a mechanism for the development of myopic choroidal neovascularization and atrophy of the outer retinal layers as seen in MMD.42 Authors have observed histological evidence of choroidal vessel narrowing and loss in highly myopic eyes.43 Parapapillary diffuse choroidal atrophy in children, a risk factor for future development of MMD, is associated with extreme thinning of the parapapillary choroid.44 Our previous studies in choroidal imaging have demonstrated:
• a stronger association of choroidal thickness with MMD compared to scleral thickness (a proxy for mechanical stretching); and
• altered choriocapillaris flow in eyes with MMD, lending further credence to this theory.10,19
Another theory holds that Bruch’s membrane production at the retro-equatorial region results in axial elongation.45 The expanding Bruch’s membrane actively compresses the choroid against the sclera which stretches passively to a certain degree.
In this manner, the Bruch’s membrane drives both the mechanical stretching and attenuation of the vascular supply to the outer retinal layers. Areas of patchy atrophy have been observed to be devoid of Bruch’s membrane, suggesting that patchy atrophy may represent expansions of Bruch’s membrane defects as the globe continues to elongate axially.
 |
While significant progress has been made in understanding the clinical features of MMD, unfortunately, no effective therapies to prevent or treat the many stages of established MMD exist.
The major exception is mCNV. The RADIANCE trial compared two different dosing regimens of ranibizumab (Lucentis, Roche/Genentech)—at day one, month one and PRN; and day one and then PRN—vs. verteporfin photodynamic therapy (Visudyne, Bausch + Lomb). Visual acuity outcomes were significantly better in both dosing regimens of ranibizumab: mean visual-acuity gain of 13.8 and 14.4 letters from baseline in the respective ranibizumab treatment groups vs. gains of 9.3 letters in the PDT group at 12 months.46
In the MYRROR study, intravitreal aflibercept (Eylea, Regeneron) was found to be effective for the treatment of mCNV, achieving an average of 13.5 letters gained at 48 weeks compared to 3.5 letters in the group that received sham injections through week 20 and then aflibercept.47 These two major trials have established intravitreal anti-VEGF injections as the first-line treatment for mCNV.48,49
What Hinders New Treatments
Several challenges hinder the development of therapies for MMD. First, the etiology of MMD is still not well understood. In particular, it is unclear whether childhood myopia will eventually develop into pathologic myopia in adulthood. Some evidence indicates that parapapillary diffuse chorioretinal atrophy in highly myopic children can be a potential biomarker for the eventual development of MMD in adulthood.50
This suggests that eyes at high risk of MMD may be fundamentally different from eyes with simple myopia. This leads to the question of whether controlling myopia progression alone is sufficient to prevent MMD in these eyes.
Second, uncertainty surrounds the optimal time for treatment, although clinicians agree that treatment should be initiated before clinically apparent and irreversible atrophy occurs. We have previously demonstrated that OCT-A may be a useful tool to identify choriocapillaris changes in eyes with no or early MMD.50 However, these findings will need to be validated in prospective studies.
That brings us to the next problem: MMD progression occurs over a prolonged period of time, thus complicating the assessment of treatment effects. Large cohorts will have to be monitored for many years, and this can only be achieved by the combined efforts of an international research consortium.
Further research will have to be undertaken to overcome these challenges but, in the meantime, efforts to reduce the overall disease burden should continue. Further advancements in imaging technology and treatment will accelerate the search for an effective MMD treatment.
References
1. Wong TY, Ferreira A, Hughes R, Carter G, Mitchell P. Epidemiology and disease burden of pathologic myopia and myopic choroidal neovascularization: an evidence-based systematic review. Am J Ophthalmol. 2014;157:9-25.
2. Holden BA, Fricke TR, Wilson DA, et al. Global prevalence of myopia and high myopia and temporal trends from 2000 through 2050. Ophthalmology. 2016;123:1036-1042.
3. Ohno-Matsui K, Kawasaki R, Jonas JB, et al. International photographic classification and grading system for myopic maculopathy. Am J Ophthalmol. 2015;159:877-883.
4. Koh V, Tan C, Tan PT, et al. Myopic maculopathy and optic disc changes in highly myopic young Asian eyes and impact on visual acuity. Am J Ophthalmol. 2016;164:69-79.
5. Samarawickrama C, Mitchell P, Tong L, et al. Myopia-related optic disc and retinal changes in adolescent children from Singapore. Ophthalmology. 2011;118:2050-2057.
6. Zheng YF, Pan CW, Chay J, Wong TY, Finkelstein E, Saw SM. The economic cost of myopia in adults aged over 40 years in Singapore. Invest Ophthalmol Vis Sci. 2013;54:7532-7537.
7. Hayashi K, Ohno-Matsui K, Shimada N, et al. Long-term pattern of progression of myopic maculopathy: A natural history study. Ophthalmology. 2010;117:1595-1611.
8. Saka N, Ohno-Matsui K, Shimada N, et al. Long-term changes in axial length in adult eyes with pathologic myopia. Am J Ophthalmol. 2010;150:562-568.
9. Lim LS, Cheung G, Lee SY. Comparison of spectral domain and swept-source optical coherence tomography in pathological myopia. Eye. 2014;28:488-491.
10. Wong CW, Teo YCK, Tsai STA, et al. Characterization of the choroidal vasculature in myopic maculopathy with optical coherence tomographic angiography. Retina. 2018 June 26 [Epub ahead of print].
11. Kaneko Y, Moriyama M, Hirahara S, Ogura Y, Ohno-Matsui K. Areas of nonperfusion in peripheral retina of eyes with pathologic myopia detected by ultra-widefield fluorescein angiography. Invest Ophthalmol Vis Sci. 2014;55:1432-1439. 12. Moriyama M, Cao K, Ogata S, Ohno-Matsui K. Detection of posterior vortex veins in eyes with pathologic myopia by ultrawidefield indocyanine green angiography. Br J Ophthalmol. 2017;101:1179-1184.
13. Shinohara K, Shimada N, Moriyama M, et al. Posterior staphylomas in pathologic myopia imaged by widefield optical coherence tomography. Invest OphthalmolVis Sci. 2017;58:3750-3758.
14. Shinohara K, Tanaka N, Jonas JB, et al. Ultrawide-field OCT to investigate relationships between myopic macular retinoschisis and posterior staphyloma. Ophthalmology. 2018 April 28 [Epub ahead of print].
15. Tokoro T. On the definition of pathologic myopia in group studies. Acta Ophthalmol Suppl. 1988;185:107-108.
16. Avila MP, Weiter JJ, Jalkh AE, Trempe CL, Pruett RC, Schepens CL. Natural history of choroidal neovascularization in degenerative myopia. Ophthalmology. 1984;91:1573-1581. 17. Curtin BJ, Karlin DB. Axial length measurements and fundus changes of the myopic eye. I. The posterior fundus. Trans Am Ophthalmol Soc. 1970;68:312-334.
18. Wong YL, Ding Y, Sabanayagam C, et al. Longitudinal changes in disc and retinal lesions among highly myopic adolescents in Singapore over a 10-year period. Eye Contact Lens. 2018 January 22 [Epub ahead of print].
19. Wong CW, Phua V, Lee SY, Wong TY, Cheung CM. Is choroidal or scleral thickness related to myopic macular degeneration? Invest Ophthalmol Vis Sci. 2017;58:907-913.
20. Fricke TR, Jong M, Naidoo KS, et al. Global prevalence of visual impairment associated with myopic macular degeneration and temporal trends from 2000 through 2050: Systematic review, meta-analysis and modelling. Br J Ophthalmol. 2018;102:855-862.
21. Lin C, Li SM, Ohno-Matsui K, et al. Five-year incidence and progression of myopic maculopathy in a rural Chinese adult population: The Handan Eye Study. Ophthalmic Physiol Opt. 2018;38:337-345.
22. Yan YN, Wang YX, Yang Y, et al. Ten-year progression of myopic maculopathy: The Beijing Eye Study 2001-2011. Ophthalmology. 2018;125:1253-1263.
23. Choudhury F, Meuer SM, Klein R, et al. Prevalence and characteristics of myopic degeneration in an adult Chinese American population: The Chinese American Eye Study. Am J Ophthalmol. 2018;187:34-42.
24. Willis JR, Vitale S, Morse L, et al. The prevalence of myopic choroidal neovascularization in the United States: Analysis of the IRIS((R)) Data Registry and NHANES. Ophthalmology. 2016;123:1771-1782.
25. Ohno-Matsui K, Lai TY, Lai CC, Cheung CM. Updates of pathologic myopia. Prog Retin Eye Res. 2016;52:156-187.
26. Axer-Siegel R, Cotlear D, Priel E, Rosenblatt I, Snir M, Weinberger D. Indocyanine green angiography in high myopia. Ophthalmic Surg Lasers Imaging. 2004;35:139-145.
27. Fang Y, Yokoi T, Nagaoka N, et al. Progression of myopic maculopathy during 18-year follow-up. Ophthalmology. 2018;125:863-877.
28. Yoshida T, Ohno-Matsui K, Yasuzumi K, et al. Myopic choroidal neovascularization: A 10-year follow-up. Ophthalmology. 2003;110:1297-1305.
29. Iacono P, Battaglia Parodi M, Papayannis A, et al. Fluorescein angiography and spectral-domain optical coherence tomography for monitoring anti-VEGF therapy in myopic choroidal neovascularization. Ophthalmic Res. 2014;52:25-31. 30. Battaglia Parodi M, Iacono P, Bandello F. Correspondence of leakage on fluorescein angiography and optical coherence tomography parameters in diagnosis and monitoring of myopic choroidal neovascularization treated with bevacizumab. Retina. 2016;36:104-109.
31. Miyata M, Ooto S, Hata M, et al. Detection of myopic choroidal neovascularization using optical coherence tomography angiography. Am J Ophthalmol. 2016;165:108-114.
32. Bruyere E, Miere A, Cohen SY, et al. Neovascularization secondary to high myopia imaged by optical coherence tomography angiography. Retina. 2017;37:2095-2101.
33. Querques G, Corvi F, Querques L, Souied EH, Bandello F. Optical coherence tomography angiography of choroidal neovascularization secondary to pathologic myopia. Dev Ophthalmol. 2016;56:101-106.
34. Querques L, Giuffre C, Corvi F, et al. Optical coherence tomography angiography of myopic choroidal neovascularisation. Br J Ophthalmol. 2017;101:609-615.
35. Ohno-Matsui K, Ikuno Y, Lai TYY, Gemmy Cheung CM. Diagnosis and treatment guideline for myopic choroidal neovascularization due to pathologic myopia. Prog Retin Eye
Res. 2018;63:92-106.
36. Ohno-Matsui K, Jonas JB, Spaide RF. Macular Bruch membrane holes in choroidal neovascularization-related myopic macular atrophy by swept-source optical coherence tomography. Am J Ophthalmol. 2016;162:133-139 e131.
37. Farinha CL, Baltar AS, Nunes SG, et al. Progression of myopic maculopathy after treatment of choroidal neovascularization. Ophthalmologica. 2014;231:211-220.
38. Spaide RF. Staphyloma: Part 1. In: Spaide RF, Ohno-Matsui K, Yannuzzi LA, eds. Pathologic Myopia. New York, NY: Springer; 2014:167-176.
39. Ohno-Matsui K. Proposed classification of posterior staphylomas based on analyses of eye shape by threedimensional magnetic resonance imaging and wide-field fundus imaging. Ophthalmology. 2014;121:1798-1809.
40. Kuo AN, Verkicharla PK, McNabb RP, et al. Posterior eye shape measurement with retinal OCT compared to MRI. Invest Ophthalmol Vis Sci. 2016;57:OCT196-203.
41. Noble KG, Carr RE. Pathologic myopia. Ophthalmology. 1982;89:1099-1100.
42. Wakabayashi T, Ikuno Y, Gomi F. Different dosing of intravitreal bevacizumab for choroidal neovascularization because of pathologic myopia. Retina. 2011;31:880-886.
43. Okabe S, Matsuo N, Okamoto S, Kataoka H. Electron microscopic studies on retinochoroidal atrophy in the human eye. Acta Med Okayama. 1982;36:11-21.
44. Yokoi T, Zhu D, Bi HS, et al. Parapapillary diffuse choroidal atrophy in children is associated with extreme thinning of parapapillary choroid. Invest Ophthalmol Vis Sci. 2017;58:901906.
45. Jonas JB, Ohno-Matsui K, Jiang WJ, Panda-Jonas S. Bruch membrane and the mechanixm of myopization: A new theory. Retina. 2017;37:1428-1440.
46. Wolf S, Balciuniene VJ, Laganovska G, et al. RADIANCE: A randomized controlled study of ranibizumab in patients with choroidal neovascularization secondary to pathologic myopia. Ophthalmology. 2014;121:682-692 e682.
47. Ikuno Y, Ohno-Matsui K, Wong TY, et al. Intravitreal aflibercept injection in patients with myopic choroidal neovascularization: The MYRROR Study. Ophthalmology. 2015;122:1220-1227. 48. Cheung CMG, Arnold JJ, Holz FG, et al. Myopic choroidal neovascularization: Review, guidance, and consensus statement on management. Ophthalmology. 2017;124:16901711.
49. Wong TY, Ohno-Matsui K, Leveziel N, et al. Myopic choroidal neovascularisation: Current concepts and update on clinical management. Br J Ophthalmol. 2015;99:289-296.
50. Yokoi T, Jonas JB, Shimada N, et al. Peripapillary diffuse chorioretinal atrophy in children as a sign of eventual pathologic myopia in adults. Ophthalmology. 2016;123:1783-1787.



