Several names have emerged in the literature to describe the varying severities of idiopathic or primary epiretinal membrane. They include cellophane maculopathy, preretinal membrane, preretinal fibrosis and macular pucker. The term epiretinal membrane is the most common in the medical lexicon and best describes the entire spectrum of this condition.
 |
ERM is a very common condition, occurring in approximately 6 to 11.8 percent of the U.S. population, but seniors are at greater risk; the incidence increases to 15.1 percent in those over age 70.1–3 Simple ERM has been described as a non-contractile membrane on the macula (Grade 1), often referred to as cellophane maculopathy. Complex ERM has been described as ERM creating striae and retinal folding with vision disturbance (Grades 2 and 3), often termed macular pucker or preretinal fibrosis.4
The pathogenesis of ERM has been controversial. This article describes three convincing arguments regarding the pathogenesis of ERM based on histologic, imaging and intraoperative observations relating to its formation.
Clinical Characteristics
The clinical characteristics of idiopathic ERM are retinal striae and folding, macular pseudohole, progressive contracture and, in rare instances, spontaneous separation (Figure 1). The ERM is almost always located in the posterior retina within the vascular arcades.
Even though the membrane may not cover the fovea, vision loss may occur due to the tractional retinal folding that extends into the fovea. The resulting metamorph-
opsia often creates a clinically significant disturbance in vision. Spontaneous separation is rare, especially in adults, and seems to be a result of posterior vitreous detachment (PVD) releasing a wrinkled or opacified posterior vitreous membrane or ERM from the retinal surface.5 This often results in improved vision, but it can be associated with bothersome premacular floaters.
 |
| Figure 1. The epiretinal membrane is difficult to discern with fundus photography (A and B), but it can easily be imaged with multicolor scanning ophthalmoscopy (C and D). |
An age-related PVD may tear, avulse or cause a dehiscence of the internal limiting membrane, allowing microglial cells access to the preretinal surface. These cells may interact with a segment of retained vitreous cortex cellular membrane on the retinal surface after a vitreoschisis or PVD.
The ERM, anchored to the underlying retina by microglial cells, undergoes contraction that creates the tangential forces that cause retinal folding, leading to macular pucker.
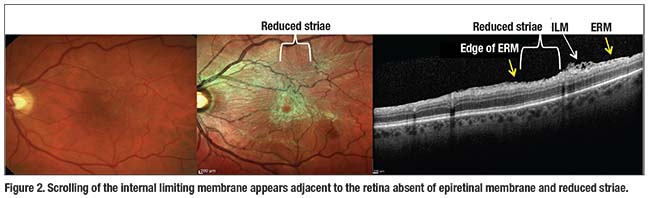
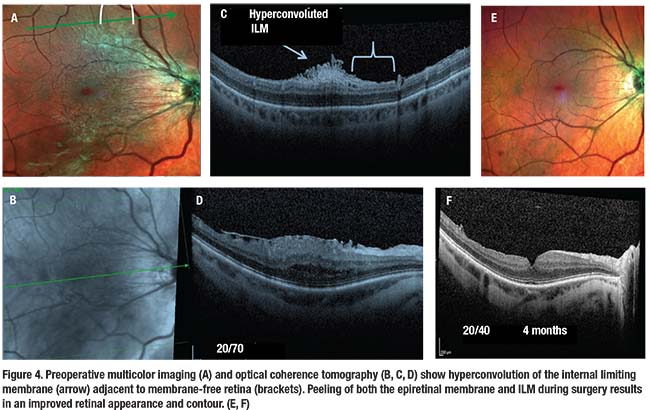
Contracture of the ERM can sometimes create an internal limiting membrane tear that scrolls into a hyperconvoluted edge, leaving a membrane-free retina without striae. This edge can easily be imaged with multicolor scanning ophthalmoscopy and correlated with SD-OCT, providing a presurgical landmark useful to peel the ERM and ILM en bloc.
Five Major Cell Types
There are five major morphologically distinguishable cells types that have been identified in pathologic specimens of ERM. They are:
• retinal pigment epithelial cells;
• macrophages;
• fibrocytes;
• fibrous astrocytes; and
• myofibroblast-like cells.
RPE cells create secondary ERM or proliferative vitreoretinopathy and are usually only found in association with retinal tears or retinal detachment. The formation of collagen and the transdifferentiation of cells to those with myofibroblastic properties appear to be the basis for the contractile properties of ERMs.6,7
 |
More recently hyalocytes, often found as resident cells in the posterior vitreous cortex, have been identified as the cells with the greatest potential to develop into myofibroblastic-like cells.8
Laminocytes have a morphology similar to hyalocytes and a distinctive laminar arrangement on the ILM with novel basement membrane production. Pathologist David Snead in the United Kingdom originally described laminocytes as the exclusive cell type in cellophane ERM, and their removal during ILM peeling may account for the success of surgery for ERM, vitreomacular traction syndrome and macular hole.9
Theories of ERM Pathogenesis
There are two commonly considered hypotheses and one newer theory, all of which have PVD as the critical event triggering ERM formation. In fact, either partial or complete PVD has been documented in 85 to 90 percent of all cases of macular pucker.3,10 ERM-like tissue, possibly opacified and wrinkled posterior hyaloid membrane, has been found in 10 percent of eyes without a PVD. This may explain the phenomenon of spontaneous ERM separation in a small group of patients.11
 |
Theory 1: Microglial Cells Migrate
Robert Foos, MD,12 originally proposed that retinal microbreaks in the ILM that develop as a result of a PVD allow microglial cells to migrate and spread onto the retinal surface. These cells pass through very tiny pores and then differentiate into fibroglial cells.
Initially described by J.R. Wolter, MD, in 1964,13 pores in the human retina were observed in histologic specimens, usually over a small vessel and accompanied by phagocytes. These phagocytes represent glial cells that stretched through the very tiny openings in the ILM, from the retina into the vitreous, or as Dr. Wolter described it, in the manner of a cat going through a small hole.
This
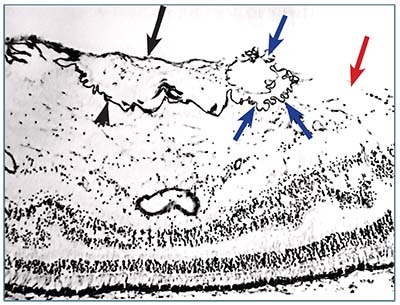 |
| Figure 3. Histology section shows a thick fibroglial epiretinal membrane (black arrow) with wrinkling (arrowhead), scrolling (blue arrows) and a large gap in the internal limiting membrane (red arrow). (Used with permission. Clarkson J et al. Am J Ophthal. 1977;84:1-17) |
appears to be a one-way journey, because these cells have not been seen to migrate in the opposite direction. The migration of these cells through the pores of the ILM may then allow considerable anchorage of the resulting membrane to the underlying retina. This ERM anchorage and contracture forms tangential traction, creating the retinal folding that results in clinically observable striae of complex ERM.
The most common argument against this theory is that microbreaks or pores are rarely observed in histologic specimens. However, although histologic sections have shown these glial cells as they migrate through the pores, these pores may spontaneously close after glial cells migrate through them.
Theory 2: Segments of Cellular Vitreous Remain on Retinal Surface
Jerry Sebag, MD,14 refined the theory originally proposed by Norman Jaffe, MD,15 and Howard Tenanbaum, MD,16 that a PVD results in segments of cellular vitreous that remain on the retinal surface. In this theory, which Dr. Sebag termed anomalous PVD, vitreous liquefaction without sufficient concurrent vitreoretinal
 |
interface dehiscence allows these segments of the vitreous to remain on the retina despite other portions completely separating from it.
Segments of vitreous that contain hyalocytes transdifferentiate into myofibroblasts after the PVD leading to ERM formation. A splitting or vitreoschisis has also been identified.17 Some studies, having failed to identify retained cortical vitreous in the location of future ERM formation,18 provide an argument against this theory.
Theory 3: Avulsion Defects
Kirk Packo, MD, based on personal intraoperative observations, has suggested that ERMs may evolve from avulsion defects primarily in the posterior paravascular retina.19 The ILM is thinnest adjacent to posterior retinal vessels and avulses during a natural or surgical vitreous separation. ERM formation then results from an upregulation of cytokines caused by this PVD-induced ILM avulsion process. Dr. Packo has observed a lack of indocyanine green staining along the paravascular retina during ERM surgery, supporting this hypothesis.
 |
The argument against this observation is that the very thin ILM in the paravascular retina may not stain with ICG or other stains, and thus the longitudinal area along the vessels may only appear to be absent of ILM.
Other authors have reported
 |
this same lack of staining pattern in the paravascular retina in histologic specimens as well.20,13 Also, ERM formation does not seem to occur following surgical peeling of ILM, although the removal of the vitreous may deprive the tissue of cellular elements necessary for ERM formation.
ILM Dehiscence
Gregory Fincham, MRCOphth, has reported on electron microscopy to demonstrate that an ILM dehiscence occurs frequently with age-related PVD, and this delaminated inner ILM layer is the posterior vitreous membrane seen clinically.21
Etienne Bovey, MD, has described that a tear, break or detachment of the ILM can occur as a result of a partial perifoveal posterior vitreous separation exerting traction on the ILM.22 According to Dr. Bovey, as the PVD continues, the edge of ILM folds inward toward an ERM present on the retinal surface; eventually the posterior vitreous separates from the ERM.
This tear or avulsion can be seen clinically during chromovitrectomy as a patch of absent ICG staining bordering an arcuate band or edge that is the folded ILM adjacent to the ERM. Histology sections from eyes enucleated for unrelated reasons have demonstrated a similar infolding or hyperconvolution of torn ILM adjacent to an ERM (Figure 3).23 Attempting to peel in this unstained area may damage the bare nerve fiber layer.22
We reported the results of spectral domain optical coherence tomography combined with multicolor imaging in eyes with ERM.24 We compared these findings to previously published histologic specimens to suggest that the ripped and scrolled ILM may be caused by the adjacent ERM contracture and only initiated by the PVD (Figure 2).
Spindle- or oval-shaped ILM dehiscences present in some eyes with ERM, and tangential traction after PVD may also cause corresponding deep lamellar-like excavations or defects in the nerve fiber layer.24
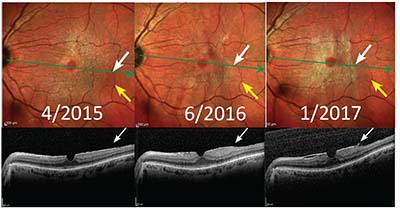 |
| Figure 6. Multicolor scans show progressive epiretinal membrane contracture (white arrows) in relation to retinal vessels (yellow arrows) with development of an ERM edge (white arrows on optical coherence tomography). |
This type of large defect, easily seen in the posterior paravascular retina using multicolor scanning ophthalmoscopy or even upon careful clinical examination, may represent a different pathogenic process because it has also been observed in high myopia without ERM.25
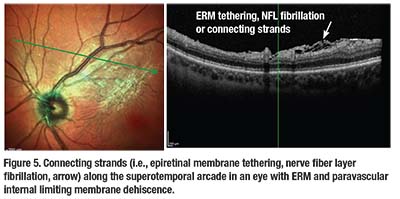
Cynthia Toth, MD, using intraoperative OCT, has described contracting ERM as a cause of tethering or fibrillation of the NFL, also termed connecting strands (Figure 5).26 These connecting strands likely result from ERM contracture causing tractional forces to the NFL, creating a schisis. Interestingly, these connecting strands have been shown to remain temporarily visible on intraoperative OCT just after ERM peeling, but retract or disappear in the days after ERM peeling.
Progression of ERM
 |
Only 9.3 percent of cellophane membranes have been reported to develop into fibrotic membranes with retinal folds.27 Nearly 29 percent of existing ERMs (both cellophane and fibrotic) and 16.1 percent of the more severe fibrotic ERMs are reported to progress over time. Most are stable and some even regress.
Although growth of the ERM has been observed, it does not typically begin as a small nidus and grow larger. Rather the ERM may begin as a nearly full-sized thin cellophane membrane with indistinct margins. This is possibly created by retained vitreous segments or after vitreoschisis splits the vitreous cortex and leaves a hyalocyte-rich cellular membrane on the retinal surface, after an age-related PVD.
Shoji Kishi, MD, in Japan has described an oval defect in the posterior hyaloid membrane that corresponds to retained membrane in the macula.11 This type of membrane is difficult to image precisely with conventional photography, especially in eyes with cataracts, and it may be challenging to accurately measure the ERM area.
Over time ERM contracture causes retinal striae and folds to develop often with displacement of retinal vessels. During this phase the ERM is more easily visible, with distinct edges, and leads to vision loss (Figure 6, page 23).28 Interestingly, this has often been referred to as progression even though the ERM area rarely increases in size. RS
REFERENCES
1. Klein R, Klein BE, Wang Q, Moss SE. The epidemiology of epiretinal membranes. Trans Am Ophthalmol Soc. 1994;92:403–425.
2. Mitchell P, Smith W, Chey T, Wang JJ, Chang A. Prevalence and associations of epiretinal membranes. The Blue Mountains Eye Study, Australia. Ophthalmology. 1997;104:1033–1040.
3. McCarty DJ, Mukesh BN, Chikani V. Prevalence and associations of epiretinal membranes in the visual impairment project. Am J Ophthalmol. 2005;140:288–294.
4. Johnson MW. Perifoveal vitreous detachment and its macular complications. Trans Am Ophthalmol Soc. 2005;103:537-567.
5. Greven CM, Slusher MM, Weaver RG. Epiretinal membrane release and posterior vitreous detachment. Ophthalmology. 1988;95:902–905.
6. Kampik A, Kenyon KR, Michels RG, et. al. Epiretinal and vitreous membranes. Comparative study of 56 cases. Arch Ophthalmol. 1981:99:1445-1454.
7. Kampik A. Pathology of epiretinal membrane, idiopathic macular hole, and vitreomacular traction syndrome. Retina. 2012;32:S194-S199.
8. Kohno RI, Hata Y, Kawahara S, et al. Possible contribution of hyalocytes to idiopathic epiretinal membrane formation and its contraction. Br J Ophthalmol. 2009;93:1020–1026.
9. Snead DR, James S, Snead MP. Pathological changes in the vitreoretinal junction 1: epiretinal membrane formation. Eye. 2008;22:1310-1317.
10. Johnson MW. Posterior vitreous detachment: Evolution and complications of its early stages. Am J Ophthalmol. 2010;149:371-382.
11. Kishi S, Shimizu K. Oval defect in posterior hyaloid membrane in idiopathic preretinal macular fibrosis. Am J Ophthalmol. 1994;118:451-456.
12. Foos RY. Vitreoretinal juncture; epiretinal membranes and vitreous. Invest Oph Vis Sci. 1977;16:416-422.
13. Wolter JR. Pores in the internal limiting membrane of the human retina. Acta Ophthalmol. 1964;42:971-974.
14. Sebag J. Anomalous posterior vitreous detachment: A unifying concept in vitreo-retinal disease. Graefe’s Arch Clin Exp Ophthalmol. 2004;242:690–698.
15. Jaffe NS. Macular retinopathy after separation of vitreoretinal adherence. Arch Ophthalmol. 1967;78:585–591.
16. Tanenbaum HL, Schepens CL, Elzeneiny I, Freeman HM. Macular pucker following retinal detachment surgery. Arch Ophthalmol. 1970;83:286–293.
17. Yamashita T, Uemura A, Sakamoto T. Intraoperative characteristics of the posterior vitreous cortex in patients with epiretinal membrane. Graefes Arch Clin Exp Ophthalmol. 2008;246:333-337.
18. Chen T, Yang C, Liu K. Intravitreal triamcinolone staining observation of residual undetached cortical vitreous after posterior vitreous detachment. Eye. 2006;20:423-427.
19. Packo K. Spontaneous avulsions of the ILM. A possible etiology of macular puckers. Paper presented at: Retina Society Annual Meeting; September 14, 2016; San Diego, Calif.
20. Wolff E. The anatomy of the eye and orbit. Philadelphia, PA, and Toronto, Canada: H.K. Lewis & Co.; 1948:95.
21. Fincham GS, James S, Spickett C, et al. Posterior vitreous detachment and the posterior hyaloid membrane. Ophthalmology. 2018;125:227-236.
22. Bovey EH, Uffer S. Tearing and folding of the retinal internal limiting membrane associated with macular epiretinal membrane. Retina. 2008;28:433-440.
23. Clarkson JC, Green WR, Massof D. A histopathologic review of 168 cases of preretinal membrane. Am J Ophthalmol. 1977;84:1-17.
24. Gross JG, Gross JS. Internal limiting membrane dehiscence visualized with multicolor scanning laser ophthalmoscopy is associated with inner retinal abnormalities in eyes with epiretinal membrane. Paper presented at: Retina Society Annual Meeting; October 7, 2017; Boston, Mass.
25. Muraoka Y, Tsujkawa A, Hata M, et. al. Paravascular inner retinal defect associated with high myopia or epiretinal membrane. JAMA Ophthalmol. 2015;133:413-420.
26. Nam DH, Desouza PJ, Hahn P, et. al. Intraoperative spectral domain optical coherence tomography imaging after internal limiting embrane peeling in idiopathic epiretinal membrane with connecting strands. Retina. 2015;35:1622-1630.
27. Fraser-Bell S, Guzowski M, Rochtchina E, et. al. Five-year cumulative incidence and progression of epiretinal membranes: The Blue Mountains Eye Study. Ophthalmology. 2003;110:34-40.
28. Lee SM, Pak KY, Kwon HJ, et.al. Association between tangential contraction and early vision loss in idiopathic epiretinal membrane. Retina. 2018;38:541-549.



