 |
However, many questions still need to be answered regarding anti-VEGF injections for DME, among them:
• Which anti-VEGF agent is most effective?
• How many injections are required?
• What role does laser play in DME management?
• Which drug is most cost effective?
• How safe is anti-VEGF treatment?
The Diabetic Retinopathy Clinical Research Network (DRCRnet) has been a leading enterprise in clinical research on diabetic retinopathy and, specifically, DME. Over the last decade, DRCRnet Protocol I evaluated ranibizumab (0.5 mg) (Lucentis, Genentech) injections vs. the “gold standard” macular photocoagulation for the treatment of DME.2 The findings of DRCRnet Protocol I clearly demonstrated superiority of ranibizumab with prompt and deferred focal laser over laser treatment alone.
The visual benefits from anti-VEGF injections for DME seen in Protocol I had been maintained through five years of follow-up.3 Subsequent clinical trials4,5 have demonstrated that bevacizumab (Avastin, Genentech) reduced vision loss due to DME, and industry-sponsored trials also found strong evidence to support the use of both ranibizumab and aflibercept (Eylea, Regeneron Pharmaceuticals) in the treatment of DME.6-10
Protocol T: Which Works Best?
To expand the knowledge base regarding effectiveness and anti-VEGF treatment strategies, the DRCRnet designed Protocol T to compare the efficacy of ranibizumab, aflibercept and bevacizumab for the treatment of DME.11 The study recruited 660 subjects with decreased vision and DME from 89 community-based and academic practices.
The study randomized the enrolled eyes into three groups receiving injections of bevacizumab (1.25 mg), ranibizumab (0.3 mg) or aflibercept (2 mg). The subjects were evaluated and treated by the standard DRCRnet anti-VEGF algorithm based on changes in visual acuity and macular thickening on optical coherence tomography. Although the study was not powered to compare safety outcomes, it did collect safety data, including ocular adverse events and systemic adverse events.
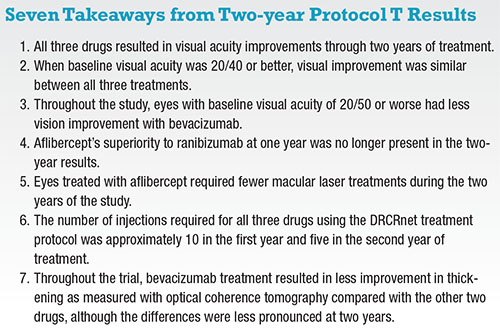
Primary outcome results at one year were published in the New England Journal of Medicine.12 A treatment course with all three drugs resulted in significantly improved visual acuity. Aflibercept resulted in the largest mean improvement of vision (18.9 letters) with ranibizumab (14.2) and bevacizumab (11.8) having less improvement at one year. The statistical difference between the acuity improvements was driven by the enrolled eyes with 20/50 or worse baseline vision. This division of the cohorts was a pre-specified analysis done by the DRCR network. Approximately half of the subjects in Protocol T had 20/40 or better vision at baseline, and these eyes showed no difference in vision outcomes between the three drugs.
 |
How Many Injections?
The DRCRnet anti-VEGF injection treatment algorithm consists of a monthly evaluation and dosing schedule with criteria for injection deferral depending on visual acuity changes and OCT measurements. This differs from other clinical trials where the dosing schedule was fixed throughout the course.8,9
The Protocol T treatment algorithm allowed for some reduction in the need for injections over time and provided an opportunity to see if any of the three drugs required fewer injections over a two-year treatment course.11 Because investigators were not masked to the treatment intervention in Protocol T, strict OCT and visual parameters were necessary to minimize investigator discretion and potential bias throughout the trial.
Protocol T follow-up showed no significant differences in the number of intravitreal injections each of the three treatment groups required, with 15 to 16 injections over the full two years and five to six in the second year alone.13 The observed reduction in the need for anti-VEGF treatment for DME was consistent with results from Protocol I.3
Certainly, there have been clinical reports of visual outcomes that were inferior to the clinical trials.14 In many cases this may be due to under- treatment or lack of adherence to an appropriate dosing program. More than 80 percent of respondents to the American Society of Retinal Specialists Preferences and Trends survey indicated that they consider alternate treatment for DME after five injections or less.15 A recent publication from the Pan-American Collaborative Retina Study reports the initial visual benefits from bevacizumab for DME were not sustained over five years.16 However, those study eyes only received a mean of 8.4 injections over five years, which is about half the injections that Protocol T eyes received over two years.
 |
The DRCRnet algorithm is not truly a “treat-and-extend” or PRN dosing regimen, so some clinicians have had difficulty in adopting it. The DRCRnet has attempted to clarify its treatment algorithm through publications and programs at meetings (Figure).17
The simplified algorithm is as follows: Once treatment with anti-VEGF injections has started, retreat every four weeks until vision and OCT are stable for two consecutive visits; after deferring injections, re-treatment begins again if edema returns or vision worsens. Even when DME is chronically persistent, the DRCRnet treatment algorithm has been shown to result in stable visual results.18
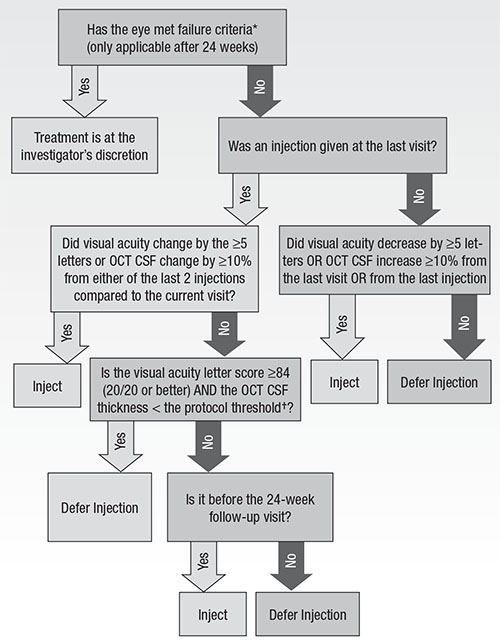 |
| Figure. Diabetic macular edema treatment with anti-VEFG during follow-up. OCT = optical coherence tomography, CSF = central subfield, DME = diabetic macular edema *Failure = failure can only be met at or after the 24-week visit IF each of the following are met: A) OCT CSF thickness ≥ eligibility threshold; B) visual acuity is 10 or more letters worse than baseline at two consecutive visits; C) DME present on clinical exam that the investigator believes is the cause of the visual acuity loss; D) complete focal/grid laser for DME has been given; E) there has been no improvement in visual acuity (>5 letters) or OCT (>10% OCT CSF thickness) since either of the last two injections; F) there has been no improvement in visual acuity (>5 letters) or OCT (>10% OCT CSF thickness) since the last focal/grid laser treatment for DME was given; and G) it has been ≥13 weeks since the last focal/grid laser treatment for DME. †Protocol threshold = >250 μm on Zeiss Stratus; ≥ 320 for men or ≥305 for women on Heidelberg Spectralis; ≥305 for men or ≥290 for women on Zeiss Cirrus. Used with permission of Massachusetts Medical Society. |
The Protocol T treatment algorithm included macular photocoagulation if, after six months of anti-VEGF treatment, DME was persistent and not improving. Additional laser treatments were permitted 13 weeks after the last laser if the DME continued to persist and untreated microaneurysms associated with the edema were present. The protocol discouraged macular photocoagulation closer than 500 μm from the macular center.
Over the two years of Protocol T, fewer eyes treated with aflibercept required macular photocoagulation; 41 percent received at least one session of focal/grid photocoagulation, vs. 52 and 64 percent for ranibizumab and bevacizumab, respectively.13
Which Is Most Cost Effective?
The three drugs studied in Protocol T have significantly differing costs. The approximate costs derived from Medicare is $1,950 per dose for aflibercept, $1,200 for ranibizumab (0.3 mg) and $50 for bevacizumab.12 The results from both years one and two of Protocol T suggest that patients with 20/40 vision or better at treatment initiation will have a similarly good chance at visual improvement with any of the three drugs.
In eyes with 20/50 or worse initial vision, aflibercept and ranibizumab may lead to a more substantial improvement in vision but have significant incremental costs over the use of bevacizumab. The cost analysis method of quality-adjusted life-years (QALY) analysis19 suggested the value of bevacizumab as a treatment for DME is substantially greater than ranibizumab or aflibercept even in eyes that benefit most from the more expensive drugs. Cost-effectiveness analyses on Protocol T results suggest that the cost of aflibercept and ranibizumab would need to be reduced by 65 to 85 percent to have similar value to bevacizumab for DME treatment.20
How Safe Is Anti-VEGF?
The incidence of adverse events in all three groups of Protocol T was consistent with previously reported safety results from major clinical trials.13 Pre-specified adverse events, including deaths and hospitalizations, were comparable between the three groups. Vascular adverse events, as defined by the Antiplatelet Trialists’ Collaboration (APTC),21 occurred more frequently in the ranibizumab group (12 percent vs. 8 percent for bevacizumab and 5 percent for aflibercept; p=0.047).13 This finding is not consistent with previous clinical trial data, especially the RISE study and DRCRnet Protocol I, in which ranibizumab treatment was associated with a lower risk of APTC events compared with control groups. Overall, intravitreal use of anti-VEGF agents has not been shown to be associated with overall mortality, cardiovascular mortality, hypertension or stroke.22
The rates of ocular adverse events were low in all three groups. One case of endophthalmitis was reported in more than 9,000 injections throughout the course of the trial. The rate of retinal tears and detachments (including tractional detachments) was less than 1 percent in each group. A rise in IOP, defined as an increase of 10 mm Hg of more from baseline, 30 mm Hg or greater at any visit or initiation of glaucoma medications or glaucoma surgery, was noted in 15 percent of the Protocol T patients.13 This will likely be an aspect of anti-VEGF injections that will receive further investigation.
Another safety issue concerns bevacizumab repackaging. The bevacizumab used in Protocol T was repackaged centrally and tested for sterility and potency into glass containers similar to those used to package the commercially available aflibercept and ranibizumab.12 However, in clinical practice the available supply of bevacizumab is generally packaged differently, often into plastic syringes, and may be less consistently safe and potent than that used in Protocol T.14,23 Although the rates of endophthalmitis associated with bevacizumab appear to be similar to commercially available ranibizumab and aflibercept,24 there have been reports of less potency in bevacizumab in plastic syringes.25 Decreased potency may cause inferior efficacy with bevacizumab.
Conclusion
Protocol T demonstrated anti-VEGF injections are an effective treatment for DME. When visual impairment is mild, all three commercially available anti-VEGF drugs improve visual acuity. In eyes that have moderate to severe visual impairment, ranibizumab and aflibercept have been shown to lead to the most visual improvement.
The cost of bevacizumab enhances its value in the management of DME compared to the other anti-VEGF agents. However, the safety and reliable potency of compounded bevacizumab may limit its utilization. Reduced anti-VEGF dosing is common in clinical practice, but lesser dosing than the DRCRnet algorithm may result in less visual improvement. RS
REFERENCES
1. Varma R, Bressler NM, Doan QV, et al. Prevalence of and risk factors for diabetic macular edema in the United States. JAMA Ophthalmol. 2014;132:1334-1340.
2. Diabetic Retinopathy Cinical Research Network. Randomized trial evaluating ranibizumab plus prompt or deferred laser or triamcinolone plus prompt laser for diabetic macular edema. Ophthalmology. 2010;117:1064-1077.
3. Elman M, Ayala A, Bressler N, et al. Intravitreal ranibizumab for diabetic macular edema with prompt versus deferred laser treatment—5-year randomized trial results. Ophthalmology. 2015;122:375-381.
4. Michaelides M, Kaines A, Hamilton RD, et al. A prospective randomized trial of intravitreal bevacizumab or laser therapy in the management of diabetic macular edema (BOLT study) 12-month data: report 2. Ophthalmology. 2010;117:1078-1086.
5. Arevalo JF, Lasave AF, Wu L, et al. Intravitreal bevacizumab plus grid laser photocoagulation or intravitreal bevacizumab or grid laser photocoagulation for diffuse diabetic macular edema: results of the Pan-American Collaborative Retina Study Group at 24 months. Retina. 2013; 33:403-413.
6. Mitchell P, Bandello F, Schmidt-Erfurth U, et al. The RESTORE study: ranibizumab monotherapy or combined with laser versus laser monotherapy for diabetic macular edema. Ophthalmology. 2011;118:615-625.
7. Korobelnik JF, Do DV, Schmidt-Erfurth U, et al. Intravitreal aflibercept for diabetic macular edema. Ophthalmology. 2014;121:2247-2254.
8. Brown DM, Schmidt-Erfurth U, Do DV, et al. Intravitreal Aflibercept for Diabetic Macular Edema: 100-week results from the VISTA and VIVID studies. Ophthalmology. 2015;122:2044-2052.
9. Nguyen QD, Brown DM, Marcus DM, et al. Ranibizumab for diabetic macular edema: results from 2 phase III randomized trials: RISE and RIDE. Ophthalmology. 2012;119:789-801.
10. Brown DM, Nguyen QD, Marcus DM, et al. Long-term outcomes of ranibizumab therapy for diabetic macular edema: the 36-month results from two phase III trials: RISE and RIDE. Ophthalmology. 2013;120:2013-2022.
11. Jampol LM, Glassman AR, Bressler NM. Comparative Effectiveness Trial for Diabetic Macular Edema: Three Comparisons for the Price of 1 Study From the Diabetic Retinopathy Clinical Research Network. JAMA Ophthalmol. 2015;133:983-984.
12. Diabetic Retinopathy Clinical Research Network. Aflibercept, Bevacizumab, or Ranibizumab for Diabetic Macular Edema. N Engl J Med. 2015;372:1193-1203.
13. Wells JA, Glassman AR, Ayala AR, et al. Aflibercept, bevacizumab, or ranibizumab for diabetic macular edema; two-year results from a comparative effectiveness randomized clinical trial. Ophthalmology. 2016 [in press]
14. Heier JS, Bressler NM, Avery RL, et al. Comparison of aflibercept, bevacizumab, and ranibizumab for treatment of diabetic macular edema: extrapolation of data to clinical practice. JAMA Ophthalmol. 2016;134:95-99.
15. Stone TW, ed. ASRS 2015 Preferences and Trends Membership Survey. Chicago, IL. American Society of Retina Specialists; 2015. Available at: https://www.asrs.org/content/documents/2015_global_trends_in_retina_survey_-_for_website.pdf. Accessed May 17, 2016.
16. Arevalo JF, Lasave AF, Wu L, et al. Intravitreal bevacizumab for diabetic macular edema: 5-year results of the Pan-American Collaborative Retina Study group. Br J Ophthalmol. doi:10.1136/bjophthalmol-2015-307950.
17. Diabetic Retinopathy Clinical Research Network Writing Committee. Rationale for the diabetic retinopathy clinical research network treatment protocol for center-involved diabetic macular edema. Ophthalmology. 2011;118:e5-e14.
18. Bressler SB, Ayala AR, Bressler NM, et al. Persistent macular thickening after ranibizumab treatment for diabetic macular edema with vision impairment. JAMA Ophthalmol. 2016;134:278-285.
19. Stein JD, Newman-Casey PA, Kim DD, Nwanyanwu KH, Johnson MW, Hutton DW. Cost-effectiveness of various interventions for newly diagnosed diabetic macular edema. Ophthalmology. 2013;120:1835-1842..
20. Bressler NM for the DRCR Network. Cost-effectiveness of aflibercept, bevacizumab and ranibizumab for diabetic macular edema. Paper presented at the American Academy of Ophthalmology Retina 2015 Sub-Specialty Day; November 14, 2015; Las Vegas, NV.
21. Antiplatelet Trialists’ Collaboration. Collaborative overview of randomised trials of antiplatelet therapy—I: Prevention of death, myocardial infarction, and stroke by prolonged antiplatelet therapy in various categories of patients. Br Med J. 1994;308:81-106.
22. Thulliez M, Angoulvant D, LeLez ML, et al. Cardiovascular events and bleeding risk associated with intravitreal antivascular endothelial growth factor monoclonal antibodies: systematic review and met-analysis. JAMA Ophthalmol. 2014;132:1317-1326.
23. Yannuzi NA, Klufas MA, Quach L, et al. Evaluation of compounded bevacizumab prepared for intravitreal injection. JAMA Ophthalmol. 2015;133:32-39
24. Gregori NZ, Flynn HW, Schwartz SG, et al. Current infectious endophthalmitis rates after intravitreal injections of anti-vascular endothelial growth factor agents and outcomes of treatment. Ophthalmic Surg Lasers Imaging Retina. 2015;46:643-648.
25. Pereboom M, Becker ML, Amenchar M, et al. Stability assessment of repackaged bevacizumab for intravitreal administration. Int J Pharm Compd. 2015; 19:70-72 .



