A 68-year-old woman presented to our clinic for a second opinion regarding areas of “geographic atrophy” in her right eye identified at her last annual diabetic eye exam. An outside retinal specialist rendered a presumptive diagnosis of atypical retinal degeneration vs. inactive central serous chorioretinopathy.
She complained of slowly progressive, diffuse blurry vision over the past year, worse in her right eye and exacerbated by dim lighting. She denied scotomas, metamorphopsia, progressive nyctalopia, or any personal or family history of retinal degeneration. Her ocular history was significant for cataracts and mild myopia without a history of myopic degeneration. She had no known history of diabetic retinopathy or macular edema.
She had been told her symptoms might be secondary to cataract with the intent to have cataract extraction soon. Her medical history included well-controlled type 2 diabetes, hypertension, hypercholesterolemia and remote bariatric surgery. She was a former smoker. A review of systems was unremarkable.
Examination Findings
Best-corrected visual acuity was 20/40 OD and 20/20 OS. Intraocular pressures were normal. We also noted bilateral nuclear sclerotic and cortical cataracts; worse in the right eye.
Fundus examination of the right eye revealed an anomalous-appearing nerve with nasal tilt and inferonasal
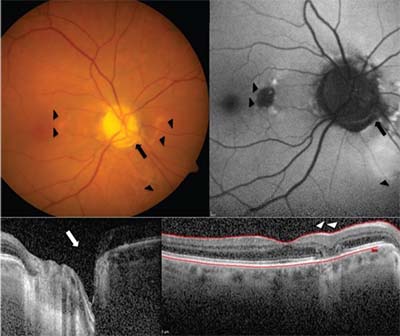 |
| Figure. Color photo of the right eye (A) shows the inferonasal optic disc pit (arrow) and pigmentary changes in the nasal macula and inferior peripapillary retina (arrowheads). Fundus auto-fluorescence (B) shows patchy hypo- and hyperautofluorescent lesion in the nasal macula corresponding to fundus photos. Optical coherence tomography (OCT) of the right eye nerve fiber layer shows inferotemporal cavitation (C, arrow), while OCT of the right macula (D) shows focal outer retinal atrophy (arrowheads). |
optic pit near the rim at 4 o’clock along with a 0.25-disc diameter patch of retinal pigment epithelium atrophy within the nasal macula (Figure A). Foveal light reflex was intact without edema or hemorrhage, and retinal vessels were normal. We noted similar retinal pigment epithelium changes in the nasal and inferonasal peripapillary retina (Figure A). Fundus examination of the fellow eye revealed a normal optic nerve except for three small areas of pigmented lattice in the periphery without holes or tears. We also noted posterior vitreous detachments in both eyes.
What the Workup Revealed
Optical coherence tomography (OCT) of the right macula demonstrated normal foveal contour and retinal laminations. A focal, well-circumscribed patch of outer retinal atrophy was present in the nasal macula, including loss of the ellipsoid zone and retinal pigment epithelium with underlying transmission defect (Figure D). No intraretinal or subretinal fluid was evident. OCT of the right optic nerve showed a pit visualized on cross section with retinal nerve fiber layer thinning in the inferonasal sector (Figure C).
Fundus autofluorescence of the right eye demonstrated focal hypoautofluorescence in the nasal macula corresponding to the area of outer retinal atrophy. Two smaller areas of hyperautofluorescence appeared superior and inferior to the atrophy. Patches of hyperautofluorescence were also located nasally and inferonasally to the disc (Figure B).
Diagnosis and Management
This patient presented with asymptomatic unilateral maculopathy in the setting of an ipsilateral optic disc pit with adjacent peripapillary retinal pigment epithelial changes. Taken together, her findings most likely represent quiescent optic pit maculopathy. Other possibilities include inactive central serous retinopathy, pattern dystrophy or a unilateral age-related macular degeneration variant. We recommended she continue regular follow-up with a retina specialist and to self-monitor with an Amsler grid. There was no contraindication to proceeding with cataract surgery as planned in the affected eye.
Discussion
Optic pits are rare, congenital defects with an estimated incidence of 1: 11,000 ophthalmology patient visits.1 They likely arise from failed closure of the embryonic fissure, although their etiology remains controversial.1–5
Optic pits present as small, hypopigmented, yellow or gray-white, oval or round excavations typically found in the inferotemporal portion of the optic disc margin. Eighty-five to 90 percent of optic pits are unilateral and the majority of affected nerves have only one pit per disc.1 The differential for optic pits includes glaucomatous optic neuropathy, optic nerve coloboma, choroidal and scleral crescent, tilted disc syndrome, circumpapillary staphyloma and hypoplastic disc.2
Optic pits are generally asymptomatic and incidentally identified on routine exam, but can be associated with an enlarged blind spot, arcuate scotoma or cecocentral scotoma corresponding with their location on the optic disc in the absence of other findings.1 Related central vision defects include decreased visual acuity, dyschromotopsia, metamorphopsia, micropsia or relative/absolute central scotomas, and are nearly exclusively associated with secondary macular pathology.1–5 In one case series, 66 percent of optic pits demonstrated macular pathology, which included schisis-like intraretinal fluid cysts, subretinal fluid and degenerative pigmentary changes thought to be remnants of longstanding serous maculopathy similar to that seen in our patient.1 In other case series, patients presenting with optic pit maculopathy typically had visual acuity worse than 20/70 in the affected eye.2
Spontaneous resolution of optic pit maculopathy is estimated to occur in 25 percent of cases, with excellent recovery of vision.2
Although recurrence of optic pit maculopathy has been reported, no triggers for initial development or recurrence are known.4 Optic pits typically become symptomatic in the third or fourth decade, but case reports demonstrate a wide range of onset from infancy to the ninth decade.4 The origin of optic pit fluid remains unclear, but leading theories implicate liquid vitreous, cerebrospinal fluid, leaky blood vessels at the base of the optic pit or fluid from the orbital space surrounding the dura.2–5
The pathogenesis for secondary maculopathy is equally controversial as only one-third of cases show a direct communication between the optic pit and macular pathology.5 Two commonly proposed mechanisms for development of maculopathy are:
Vitreous traction, made worse by progressive vitreous liquefaction in the third and fourth decade.2-5
As a result of the latter, most treatment options are aimed at inducing posterior vitreous detachment, reducing traction and disrupting communication between the optic pit and macula. However, due to the lack of comparative treatment studies of this rare phenomenon, no established treatment guidelines exist, although surgical intervention is generally recommended with active serous maculopathy due to the poor visual prognosis.
Acceptable surgical options, often performed in combination, include pars plana vitrectomy, internal limiting membrane peel, gas tamponade (C3F8 or SF6), laser
 |
photocoagulation applied to the temporal margin of the disc and macular buckling.4 Due to the paucity of supporting literature, prophylactic treatment is generally not recommended for incidentally identified optic pits without maculopathy or for quiescent maculopathy without fluid.4
When evaluating patients with retinal pathology, maintaining a systematic approach to the dilated fundus exam, including careful evaluation of the optic disc, is prudent. Although rare, optic pit maculopathy should be included in the differential for serous maculopathy at any age, but particularly in patients in the third and fourth decade and for peripapillary atrophic changes associated with an anomalous disc. Although prognosis is poor without intervention, case series of surgical interventions have shown excellent results and visual recovery, although long-term data and comparative studies are needed. RS
REFERENCES
1. Kranenburg EW. Crater-like holes in the optic disc and central serous retinopathy. Arch Ophthalmol. 1960:64:912-924.
2. Georgalas I, Ladas I, Georgopoulos G, et al. Optic disc pit: a review. Graefes Arch Clin Exp Ophthalmol. 2011; 249(8):1113-1122.
3. Jain N, Johnson MW. Pathogenesis and treatment of maculopathy associated with cavitary optic disc anomalies. Am J Ophthalmol. 2014;158:423-435.
4. Moisseiev E, Moisseiev J, Loewenstein A. Optic disc pit maculopathy: when and how to treat? A review of the pathogenesis and treatment options. Int J Retina Vitreous. 2015;1:13. eCollection.
5. Roy R, Waanbah AD, Mathur G, et al. Optical coherence tomography characteristics in eyes with optic pit maculopathy. Retina. 2013;33:771-775.



