| ABOUT THE AUTHORS | |
 | Drs. Payne (top) and Clark are vitreoretinal specialists at Palmetto Retina Center and are associate clinical professors at the University of South Carolina School of Medicine, Columbia. DISCLOSURES: Dr. Payne has received grants from OD-OS, Genentech and Regeneron. He is the principal investigator of the TREX-DME trial. Dr. Clark has received grants from Genentech, OD-OS, Regeneron, Allergan and Santen, and is a consultant to Bayer, Genentech, Regeneron and Santen. |
 | |
Recently, a computer-guided laser photocoagulation system that utilizes real-time imaging and tracking has emerged to enhance the safety and accuracy of focal laser treatment and improve visual outcomes. This article describes the evolution of computer-guided focal laser therapy for the treatment of DME and explores the clinical experience with this approach to date.
Tracking Avoids Near Misses
Focal/grid laser therapy has been traditionally performed with a slit-lamp delivery system, and requires the treating physician to localize and treat all microaneurysms in the areas of edema. This treatment can sometimes be problematic due to inadequate visualization of all leaking microaneurysms and an inability to accurately target the lesions on a living, moving fundus. Near misses of laser treatments can cause collateral damage, which can lead to macular scarring and scotomas. Additionally, physician variability makes it difficult to analyze data that utilize traditional focal/grid laser therapy in clinical trials.Advances in computer-assisted tracking systems have enabled the development of a computer-guided, laser-targeting device. The navigated laser photocoagulator, also known as the Navilas laser system (OD-OS GmbH), utilizes a retinal eye-tracking laser delivery system with integrated digital fundus imaging (Figure 1). Registered image overlays allow the physician to map and target microaneurysms and provide laser stabilization through real-time fundus tracking.
The instrument is the first to use real-time retinal tracking and takes approximately 25 images per second in order to maintain registration during planning and treatment.1 The Navilas photocoagulator was designed to improve the accuracy of laser photocoagulation and provide the ability to localize all microaneurysms and other lesions that require accurate treatment while overcoming the difficulties of slit-lamp based delivery.
 | Figure 1. The Navilas laser photocoagulator is a computer-navigated laser system, which utilizes a retinal eye-tracking laser delivery system with integrated digital fundus imaging. Figure 2. Clinical example of a Navilas focal laser session for diabetic macular edema, where microaneurysms are targeted on the fluorescein-angiogram image (A and B), and treatment effect is immediately visible with faint gray laser burns (C). |
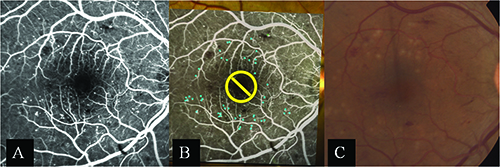 | |
The standard Navilas system uses a diode-pumped, solid-state laser (532 nm) for single-spot, grid and panretinal photocoagulation. Unlike conventional and semi-automated delivery systems, the Navilas laser is fundus-camera based. This allows for reflex-free visualization of larger areas of the retina during treatment, and the infrared light used during treatment is more comfortable for the patient. Figures 2A and B demonstrate a typical laser session in which microaneurysms are targeted on the digital display. Figure 2C displays the treatment effect immediately following treatment.
A newer model of the Navilas, which received Food and Drug Administration approval in February 2015, uses a 577-nm wavelength yellow laser to minimize laser scatter and maximize tissue absorption. Targeted, computer-guided laser therapy should allow for less energy to be delivered and less uptake from macular pigments, leading to less collateral damage to the photoreceptors. This latest version of the Navilas laser system also allows for a navigated microsecond pulsing option for subthreshold delivery.
The digital display enables the physician to apply evenly spaced confluent grids of laser and document exactly where treatments are applied. This function is invaluable when giving subthreshold treatment because the laser burns are not visible clinically.
Clinical Trial Results
The Navilas laser has been shown to have greater accuracy than conventional focal/grid laser treatment. In 2011, Igor Kozak, MD, and colleagues assessed the accuracy of 400 random focal spots from the Navilas system performed on 86 patients and found that the laser achieved a 92- percent microaneurysm hit rate. This was statistically significant when compared to the 72-percent accuracy rate of conventional focal/grid laser treatment in the control group (p <0.01).2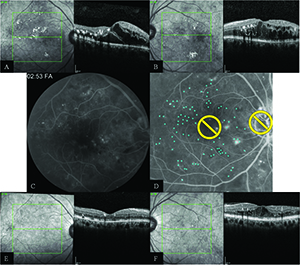 |
| Figure 3. Imaging of a subject enrolled into the TREX-DME trial. Both eyes received four monthly injections of intravitreal ranibizumab and then underwent a treat-and-extend regimen of ranibizumab based on disease activity. The right eye also received navigated laser therapy at month one and again at every three months if leaking microaneurysms were present. Figures A and B (screening) demonstrate diffuse center-involving diabetic macular edema in both eyes. Fluorescein angiography shows numerous areas of leakage on fluorescein angiography in the right eye (C) that were targeted for treatment (D). At one year (E and F), the retinal anatomy showed dramatic improvement, particularly in the right eye that received navigated laser therapy. |
| The Evolution of Computer Navigation for Lasers |
| Physicians have used light energy to treat retinal diseases since Gerd Meyer-Schwickerath, MD, began using sunlight and xenon arc coagulation in the 1950s.6,7 Laser pioneers such as Charles Campbell, MD, and H. Christian Zweng, MD,8,9 experimented with the ruby laser for the treatment of retinal conditions. Soon, Francis L’Esperance Jr., MD, and Arnall Patz, MD, worked on a more precise way to deliver light energy to the retina with the argon laser.10,11 While physicians were still establishing the pathogenic mechanisms of diabetic retinal disease, many began realizing that direct treatment of microaneurysms with laser photocoagulation often had a beneficial effect. We still do not completely understand the exact mechanisms of action for laser photocoagulation, but one theory holds that decreased edema may result from direct closure of leaking microaneurysms.12 Investigators have suggested that photocoagulation decreases edema because it reduces retinal tissue, leading to decreased retinal blood flow because of alterations in autoregulation.13,14 Others have hypothesized that reduced retinal blood flow and edema is due to improved oxygenation after laser treatment.4 Studies have shown that photocoagulation alters the retina pigment epithelial cells that produce proangiogenic cytokines like vascular endothelial growth factor.15,16 ETDRS Shows the Way Forward In 1985, the Early Treatment Diabetic Retinopathy Study (ETDRS) established a clear beneficial effect of focal/grid laser photocoagulation for diabetic macular edema (DME).17 Focal/grid laser involved two strategies within areas of retinal thickening: Direct treatment to microaneurysms within two disc diameters of the center of the macula. Scatter or grid treatment approximately two burn widths apart to areas of diffuse thickening to reduce the risk of moderate vision loss in DME and mild to moderate nonproliferative diabetic retinopathy (NPDR) regardless of baseline visual acuity by approximately 50 percent at three years.18 Additionally, 17 percent of those treated with focal laser had a three-line improvement in vision at five years vs. 5 percent of those not treated with laser.19 Patients with the greatest visual benefit after treatment had clinically significant macular edema at baseline.17–19 Retina specialists adopted a modified laser approach utilizing less-intense burns, with results similar to the original ETDRS protocol.12 Investigators later compared grid laser photocoagulation, termed mild macular grid technique, with the modified ETDRS treatment consisting of both focal closure of microaneurysms and application of light grid photocoagulation. They reported that grid treatment alone was not as effective as the modified ETDRS treatment.20 Also, eyes in the modified ETDRS group experienced a slightly greater reduction in retinal thickening and a trend toward a slightly better visual acuity outcome.20 But as lasers evolved, so too did the realization that macular laser treatment can cause retinal scarring, which can enlarge over time and cause scotomas. This finding was a motivating factor for the development of computer-assisted retinal-tracking systems. Laser Therapy vs. Intravitreal Therapy Focal/grid laser therapy continued to serve as the standard of care for primary treatment of DME until the arrival of intravitreal therapies. Before anti-VEGF medications, preservative-free intravitreal triamcinolone acetonide (IVTA) was used to decrease macular edema by inhibiting VEGF expression and other pro-inflammatory cytokines that cause vascular permeability. In 2008, the Diabetic Retinopathy Clinical Research Network (DRCR.net) compared laser photocoagulation with two doses of IVTA.21 Despite early results favoring 4 mg IVTA at four months, the DRCR.net found that IVTA was not superior to focal/grid photocoagulation in terms of visual acuity improvement or optical coherence tomography findings at two years. Additionally, rates of intraocular pressure elevation and cataract formation were higher in the IVTA groups than in the laser groups.21 Growing evidence has shown that anti-VEGF treatments are more efficacious and safer than focal laser alone for DME. In early 2015, the DRCR.net researchers published five-year data that concluded that focal/grid laser treatment at the initiation of ranibizumab (Lucentis, Genentech) therapy is no better than deferring laser treatment for at least six months in eyes with center-involving DME. Additionally, they reported that while approximately half of the eyes in the deferred laser treatment group avoided laser for at least five years, these eyes often required more injections. The eyes assigned to the prompt laser group needed fewer injections (median difference of four, or approximately 25 percent fewer injections) than the deferred laser group.21 This finding suggested that focal/grid treatment may decrease treatment burden over a long period of time. |
TREX DME Trial
The investigator-sponsored treat-and-extend (TREX) DME trial (NCT01934556) utilizing the Navilas as adjunct treatment for DME is underway. This multicenter, prospective trial utilizing ranibizumab compares a treat-and-extend algorithm with and without angiography-guided macular laser to monthly dosing for center-involving DME. To date, 150 eyes have been enrolled into three well-balanced groups—monthly cohort, treat-and-extend (TREX cohort) and treat-and-extend with angiography-guided laser photocoagulation (GILA cohort).5At six months, mean visual acuity improved by 7.8 letters and mean retinal thickness improved 89.7 µm on optical coherence tomography (OCT) in the monthly group. This can be compared to an improvement of 9.2 letters and 130.4 µm in the TREX cohort and 8.3 letters and 140.8 µm in the GILA group. The primary endpoint is the change in best-corrected visual acuity at two years, and the one-year outcomes will be available in 2016.
Figure 3 depicts a clinical example of a patient in the TREX DME trial with bilateral center-involving DME who had both eyes enrolled into the study. The left eye was randomized into the TREX cohort, in which eyes receive four monthly injections of ranibizumab and then begin a TREX regimen of intravitreal ranibizumab based on disease activity. The right eye was randomized into the GILA cohort, where subjects follow the same ranibizumab treatment scheme but have fluorescein angiography-guided macular laser at month one and then again every three months if leaking microaneuryms are present.
At baseline, both eyes had severe nonproliferative diabetic retinopathy and diffuse DME. The screening visual acuity was 20/50 in both eyes and central foveal thickness measurements on spectral-domain OCT were 516 µm in the right eye and 548 µm in the left eye. At one year, the eye receiving guided laser therapy showed greater improvement in vision (+23 letters vs. +10 letters) and a more significant decrease in foveal thickness (-275 µm vs. -148 µm). Additionally, the eye that received guided laser treatment required five fewer injections at one year. While not every patient responds to laser treatment as dramatically, this case illustrates that guided-laser therapy for DME can improve visual and anatomical outcomes and decrease treatment burden.
Further clinical research, such as the TREX DME trial, will determine how effective computer-navigated laser therapy can be. This technology pushes the boundary of our understanding of DME treatment and provides alternative strategies for management of this disease. RS
References
1. Kernt M, Cheuteu R, Vounotrypidis E, et al. Focal and panretinal photocoagulation with a navigated laser (NAVILAS). Acta Ophthalmol. 2011;89:e662-664.2. Kozak I, Oster SF, Cortes MA, et al. Clinical evaluation and treatment accuracy in diabetic macular edema using navigated laser photocoagulator NAVILAS. Ophthalmology. 2011;118:1119-1124.
3. Neubauer A, Langer J, Leigl R, et al. Navigated macular laser decreases retreatment rate for diabetic macular edema: a comparison with conventional macular laser. Clin Ophthalmol. 2013;7:121-128.
4. Liegl R, Langer J, Seidensticker F, et al. Comparative evaluation of combined navigated laser photocoagulation and intravitreal ranibizumab in the treatment of diabetic macular edema. PLoS One. 2014;9:e113981.
5. ClinicalTrials.gov. A safety and efficacy trial of a treat and extend protocol using ranibizumab with and without laser photocoagulation for diabetic macular edema (TREX-DME). Available at: https://www.clinicaltrials.gov/ct2/show/NCT01934556?term=TREX&rank=3. Accessed October 14, 2015.
6. Meyer-Schwickerath G. Light coagulation: a method for treatment and prevention of the retinal detachment. Albrecht Von Graefes Arch Ophthalmol. 1954;156:2-34.
7. Wolfensberger TJ, Hamilton AM. Diabetic retinopathy—an historical review. Semin Ophthalmol. 2001;16:2-7.
8. Campbell CJ, Rittler MC, Koester CJ. The optical maser as a retinal coagulator: an evaluation. Trans Am Acad Ophthalmol Otolaryngol. 1963;67:58-67.
9. Zweng HC, Flocks M, Kapany NS. Experimental laser photocoagulation. Am J Ophthalmol. 1964;58:353-362.
10. L’Esperance FA. An ophthalmic argon laser photocoagulation system: design, construction, and laboratory investigations. Trans Am Ophthalmol Soc. 1968;66:827-904.
11. Patz A, Maumenee AE, Ryan SJ. Argon laser photocoagulation: advantages and limitations. Trans Am Acad Ophthalmol Otolaryngol. 1971;75:569-579.
12. Shah AM, Bressler NM, Jampol LM. Does laser still have a role in the management of retinal vascular and neovascular diseases. Am J Ophthalmol. 2011;152:332-339.
13. Wilson DJ, Finkelstein D, Quigley HA, Green WR. Macular grid photocoagulation: an experimental study on the primate retina. Arch Ophthalmol. 1988;106:100-105.
14. Arnarsson A, Stefansson E. Laser treatment and the mechanism of edema reduction in branch retinal vein occlusion. Invest Ophthalmol Vis Sci. 2000;41:877-879.
15. Ogata N, Ando A, Uyama M, Matsumura M. Expression of cytokines and transcription factors in photocoagulated human retinal pigment epithelial cells. Graefes Arch Clin Exp Ophthalmol. 2001;239:87-95.
16. Ogata N, Tombran-Tink J, Jo N, Mrazek D, Matsumura M. Upregulation of pigment epithelium-derived growth factor after laser photocoagulation. Am J Ophthalmol. 2001;132:427-429.
17. Early Treatment Diabetic Retinopathy Study Research Group. Photocoagulation for diabetic macular edema: Early Treatment Diabetic Retinopathy Study report number 1. Arch Ophthalmol. 1985;103:1796-1806.
18. Early Treatment Diabetic Retinopathy Study Research Group. Photocoagulation for diabetic macular edema: Early Treatment Diabetic Retinopathy Study report no. 4. Int Ophthalmol Clin. 1987;27:265-72.
19. Early Treatment Diabetic Retinopathy Study Research Group. Early photocoagulation for diabetic retinopathy. ETDRS report no. 9. Ophthalmology. 1991;98(suppl):767-785.
20. Writing Committee for the Diabetic Retinopathy Clinical Research Network. Comparison of the modified Early Treatment Diabetic Retinopathy Study and mild macular grid laser photocoagulation strategies for diabetic macular edema. Arch Ophthalmol. 2007;125:469-480.
21. Diabetic Retinopathy Clinical Research Network. A randomized trial comparing intravitreal triamcinolone acetonide and focal/grid photocoagulation for diabetic macular edema. Ophthalmology. 2008;115:1447-1459.



