That work led her to start the company Bionic Sight, which formed a strategic collaboration last month with Applied Genetic Technologies Corp. (AGTC), a clinical stage biotechnology company with a pipeline of six gene therapies in ophthalmology. The idea is to use AGTC’s knowledge of gene therapy and Bionic Sight’s neuroprosthetic device to stimulate the remaining healthy cells in the retina with the retina’s neural code to restore normal neural signaling in patients with visual deficits or blindness due to retinal disease.
No Surgery Required
A key part of what makes this treatment different from prosthetic treatments like the Argus II epiretinal prosthesis (Second Sight Medical Products) and others in development is that it does not require any surgery. This is because it doesn’t use electrodes to stimulate cells, but instead uses optogenetics, which only requires an injection into the patient’s eye. A vector carrying the gene for an optogenetic protein is injected into the eye and taken up by the ganglion cells, and the optogenetic protein can then be used to drive the cells to send signals to the brain.
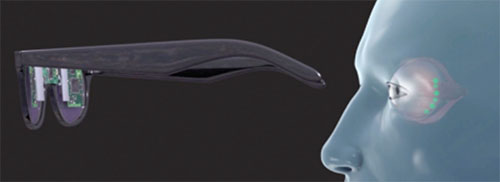 |
| A conceptual drawing of the Bionic Sight neuroprosthetic device that is built into a pair of glasses or goggles and relays signals to retinal ganglion cells injected with a transgene to restore vision in people with damaged photoreceptors. |
The neuroprosthetic treatment consists of two parts: the device and the optogenetic protein. “The device takes images in and converts them into the retina’s code,” Dr. Nirenberg says. “It does this by performing a transformation on them that turns them into the same patterns of action potentials that the normal retina would produce; that is, it converts them into the language of the brain.”
The signals are then sent to the ganglion cells, which use the optogenetic protein to send the signals forward, so they reach the brain.
Bypassing the Problem
The concept is not to target damaged photoreceptors. “Instead it is to take patients with retinal degenerative diseases, who have very damaged photoreceptors, and bypass the problem by going right to the ganglion cells,” Dr. Nirenberg says. The ganglion cells then send the code directly on to the brain. AGTC would develop adeno-associated virus-based gene therapies to deliver those optogenetic vectors into the ganglion cells.
This approach is unique because typical gene-replacement therapy requires accurate identification of the damaged gene and then replacing it with new genetic material. “With this approach, it doesn’t matter which gene caused the problem; as long as the patient still has functioning ganglion cells, we’ll jump right over the damaged photoreceptors and go right to the final-stage output cells,” Dr. Nirenberg says. However, this approach won’t work in glaucoma because the ganglion cells are damaged.
The research with the device is still in the preclinical stage, but Dr. Nirenberg says the team of investigators is finishing the remaining safety studies and is in the process of compiling a pre-investigational new drug application for the Food and Drug Administration. RS




